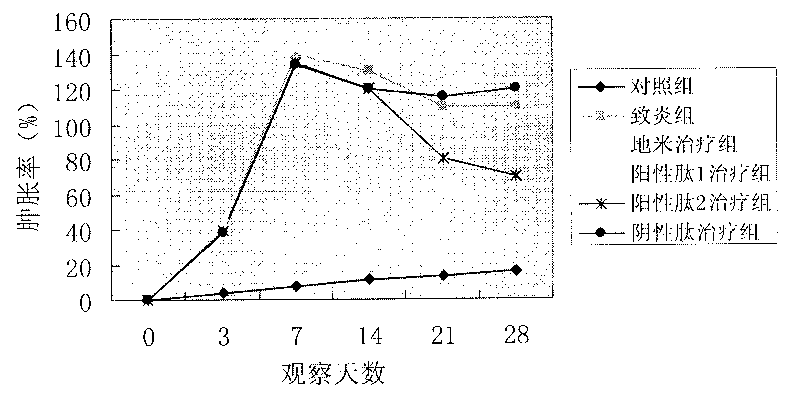
Anti-inflammatory peptide with membrane penetration effect
A short peptide, membrane-penetrating technology, applied in the field of medicine, can solve problems such as physiological dysfunction, and achieve the effect of short peptide sequence and easy synthesis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] The operating steps of embodiment 1, Lys (D-Pro) ValCit preparation method
[0024]The main operation steps of solid-phase peptide synthesis are as follows: 1. Resin swelling: Put Wang-Resin resin into a reaction tube, add DMF (15ml / g) for 30min. 2. Deprotection: remove DMF, add 20% piperidine DMF solution (15ml / g) for 5min, remove and add 20% piperidine DMF solution (15ml / g) for 15min. 3. Detection: Take out the piperidine solution, take more than a dozen resins, wash them three times with ethanol, add ninhydrin, KCN, and phenol solution one drop each, heat at 105°C-110°C for 5 minutes, and turn dark blue to indicate a positive reaction. 4. Washing: DMF (10ml / g) twice, methanol (10ml / g) twice, DMF (10ml / g) twice. 5. Condensation: Add a three-fold excess of protected amino acids and a three-fold excess of activator HBTU, dissolve them with as little DMF as possible, add to a reaction tube, immediately add a ten-fold excess of NMM, and react for 30 minutes. 6. Washin...
Embodiment 2
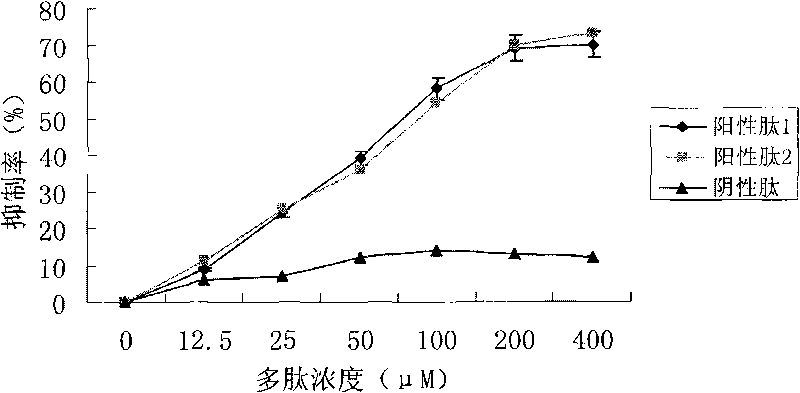
[0025] Example 2, ELISA method to detect polypeptide binding to nuclear factor-κB p65 subunit and nuclear factor-κB cis-acting element Competitive Inhibition Experiment
[0026] The synthesis of the peptide sequence was synthesized by Shanghai Qiangyao Biotechnology Co., Ltd., with a purity of >95%. On a 96-well enzyme-linked plate coated with nuclear factor-κB cis-acting elements (purchased from Mercury TM Add 150 μl / well of transcription factor blocking solution to Transfactor p65kits), incubate at room temperature for 15 minutes; discard the transcription factor blocking solution, add 50 μl / well of detection samples diluted with transcription factor blocking solution, and the samples contain 10 ng / μl of p65 standard protein as positive Control, p65 standard protein containing 10ng / μl was added with serial concentrations of peptides at the same time, p65 standard protein containing 10ng / μl was added with the same series of negative peptides at the same time, incubated a...
Embodiment 3
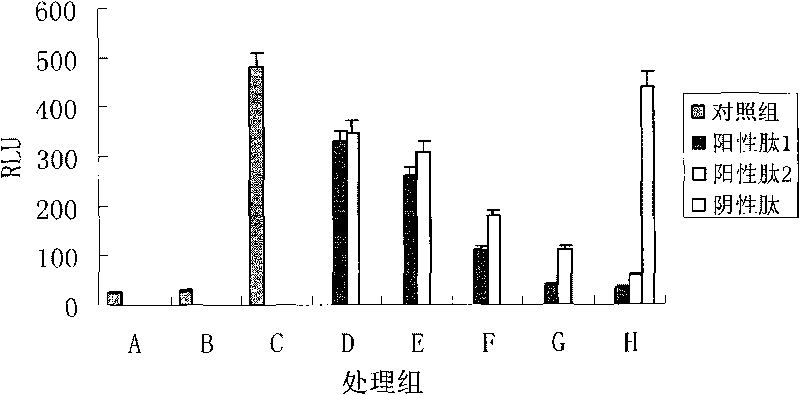
[0028] Example 3. Inhibition experiment of polypeptide on expression of nuclear factor-κB responsive reporter gene.
[0029] RAW 264.7 cells were cultured in 1640 medium, and the cell density was adjusted to 0.5-2.5×10 the day before transfection 5 Cells / mL, overnight culture; take 1mL of overnight culture cells or 0.5×10 5 The amount of cells per well was added to a 24-well culture plate; 3 μL GeneJuice transfection reagent was added to 100 μL serum-free 1640 medium, vortexed to mix, and left at room temperature for 5 min; 1 μg p4kB-Luc plasmid was added to the GeneJuice / 1640 medium mixture, gently Mix by blowing and blowing, and place at room temperature for 5-15 minutes; add the above mixture dropwise to the complete medium, shake gently to mix the mixture and cells thoroughly; culture in a 5% CO2 incubator at 37°C; after 5 hours of transfection, use complete The culture medium was exchanged for 1 h, and then the cells were washed 3 times with PBS, and finally the cells ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com