Polypeptide targeting egfr to inhibit egf to promote tumor cell proliferation
A cell proliferation and targeting technology, applied in the field of biomedicine, can solve the problems of high production cost of monoclonal antibodies, many adverse reactions of patients, strong toxic and side effects of small molecule inhibitors, etc., and achieves easy to achieve large-scale production and short peptide sequences. , the effect of low production cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
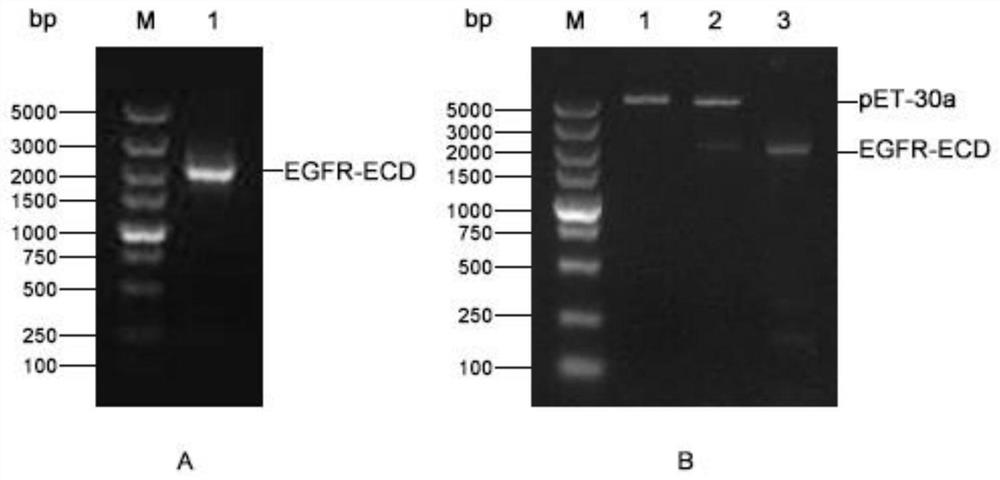
[0023] Embodiment 1: Construction of pET-30a / EGFR-ECD expression vector
[0024] The DNA sequence encoding the 25th to 645th amino acids (aa25-645) of the EGFR extracellular domain (EGFR-ECD, EGFR extracellular domain) fragment was extracted from Genebank (https: / / www.ncbi.nlm.nih.gov, login No.: CCDS5514.1), as shown in SEQ.ID.NO.2, and design PCR primers based on it:
[0025] Forward primer (SEQ.ID.NO.3): 5'-GCTGATATC GGATCC ATGCTGGAGGAAAAGAAAGT-3'
[0026] and reverse primer (SEQ.ID.NO.4): 5'-ACGGAGCTC GAATTC TCAGGACGGGATCTTAGG-3', the underlined parts are the restriction sites BamHI and EcoRI at both ends respectively. The PCR product of the target gene EGFR-ECD and the vector pET-30a (Novagen, #69909-3) were digested with BamHI and EcoRI at 37°C for 1 h, then ligated with T4 DNA ligase at 16°C for 12 h. Transform the ligation product into DH5α competent cells (full gold, CD201), wait for a single colony to grow on the kanamycin (50 µg / mL) resistant LB plate, pick a ...
Embodiment 2
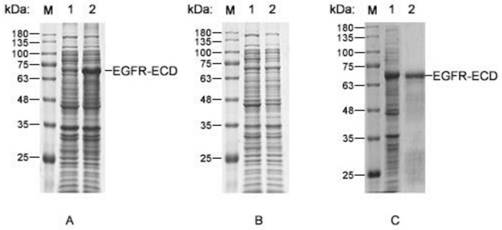
[0028] Example 2: Induced expression and refolding purification of EGFR-ECD
[0029] Induced expression of EGFR-ECD: Transform the successfully constructed recombinant plasmid pET-30a / EGFR-ECD into the host strain BL21(DE3) (full gold, CD601), use the kanamycin resistance plate to screen recombinants, and pick single The colonies were cultured in LB liquid medium containing kanamycin for 10 h. The culture was inoculated in LB liquid medium at a volume ratio of 1:100, and cultured to OD at 37°C with vigorous shaking 600 =0.5-0.6, add 0.1 M IPTG to make the final concentration 0.5 mM, and induce at 25°C for 4 h.
[0030] Verification of the expression of the target protein: Centrifuge the bacterial solution obtained from the above induced expression at 5000 rpm for 10 min, remove the supernatant, and resuspend the bacteria in the lysate (50 mM Tris -HCl pH7.5, 500 mM NaCl, 10% glycerol, 1% TritonX-100, 1 mM protease inhibitor PMSF, 1 mg / mL lysozyme), placed on ice and sonicate...
Embodiment 3
[0034] Example 3: Phage display panning for biologically active peptides specifically binding to EGFR
[0035] Immobilization of target molecules: Add 100 µL of target molecule EGFR-ECD solution with a concentration of 100 µg / mL (the base solution is 0.1 MpH7.4 TBS) into a 96-well plate, place on a shaker and vibrate slightly, and place in a humidified container Incubate overnight at 4°C. The target molecule solution was removed and washed 6 times with TBS-T (50 mM Tris-HCl pH7.5, 150 mM NaCl, 0.1% [v / v] Tween-20). Finally, block with blocking solution (0.1 M NaHCO3 pH8.6, 5 mg / mL BSA, 0.02% NaN3) for 1 h.
[0036] Binding of phage random peptide library to target molecules: remove the blocking solution and wash 10 times with TBS-T (containing 0.1% [v / v] Tween-20). Phage library or amplified phage (purchased from NEB (Beijing) Co., Ltd., E8111, Ph.D.-12 Phage Display Peptide Library) diluted with TBS-T (containing 0.1% [v / v] Tween-20) , the titer of phage was 10 9 -10 11 ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com