An affinity peptide for detecting human cancer cells and its application
A cancer cell and polypeptide technology, applied in the field of affinity peptide detection of human cancer cells, can solve the problems of complicated technology, high rate of misdiagnosis and missed diagnosis, and long time consuming.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0103] Example 1. Detection of affinity peptides for human cancer cells
[0104] 1. Design of affinity peptide
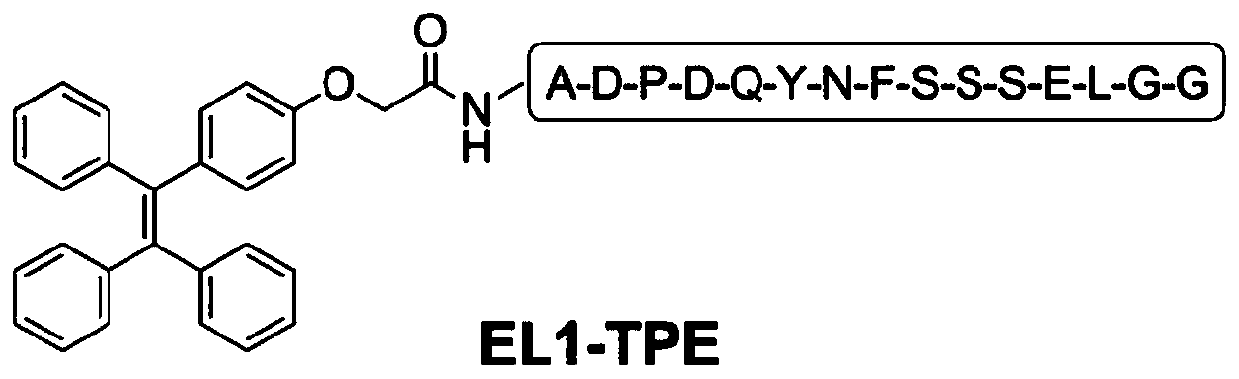
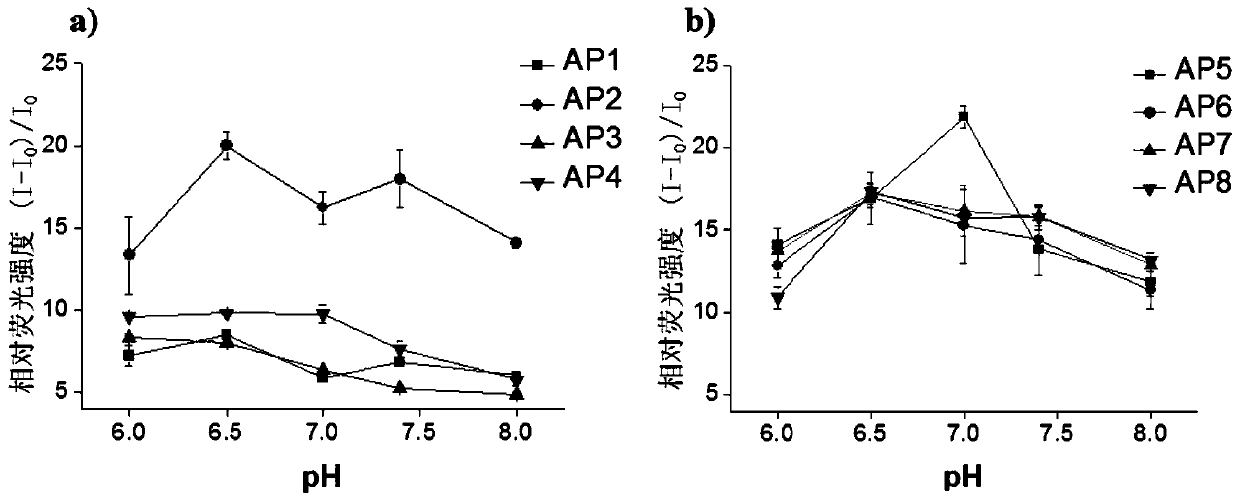
[0105] After a lot of pre-experiments and functional verification, a group of functional polypeptides that specifically bind to the first extracellular region of the LAPTM4B protein in human liver cancer and other cancer cells (EL1 for short) were designed, named AP1, AP2, AP3, and AP4 respectively. , AP5, AP6, AP7 and AP8, EL1 and the sequences of eight functional polypeptides are shown in Table 1.
[0106] The letters represent amino acids as follows: alanine A, aspartic acid D, proline P, glutamine Q, tyrosine Y, asparagine N, phenylalanine F, serine S, glutamic acid E, Leucine L, Glycine G, Isoleucine I, Arginine R, Valine V, Lysine K, Threonine T.
[0107] Table 1. EL1 sequence and polypeptide sequence
[0108]
[0109]
[0110] 2. Preparation of fluorescent probes for peptide screening
[0111] Use the FMOC solid-phase peptide synthesis method to syn...
Embodiment 2
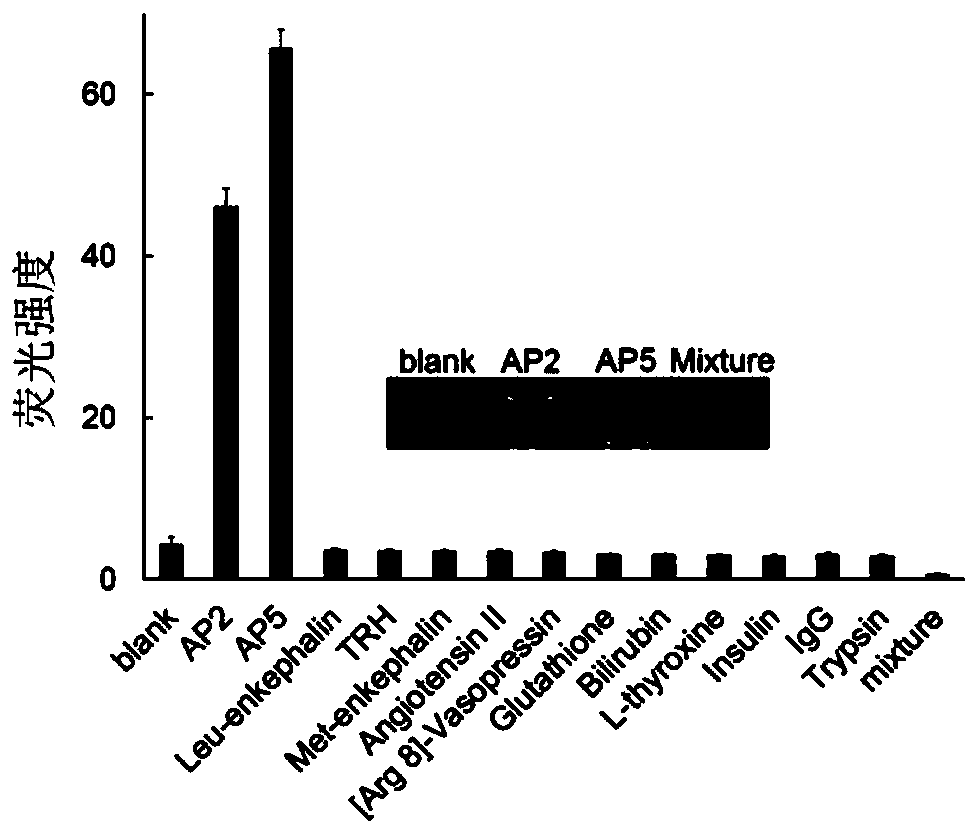
[0151] Example 2, Application of Affinity Peptides in Detecting Human Cancer Cells
[0152] 1. Preparation of AP2-Cy7 and AP5-Cy7 conjugates
[0153] 1. Dissolve 100mg of IR-780 iodide and 18mg of 3-azidopropylamine in 15mL of DMF, react at 80°C for 16 hours, distill off the solvent under reduced pressure, and purify the residue by silica gel column chromatography (dichloromethane / methanol, 20:1 , v / v), to obtain 47mg dark blue solid, which is the azide Cy7. The structural formula and mass spectrometric identification results of azide Cy7 are shown in Figure 4 .
[0154] 2. Polypeptides AP2 and AP5 with alkyne groups at the end were directly synthesized on Wang resin by FMOC solid-phase peptide synthesis method. The specific method is: start to synthesize the polypeptide sequence from the C-terminus, and finally connect propargylglycine to the N-terminus. After the Kaiser reagent detects that the reaction is complete, the terminal FMOC protecting group is removed, and then...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com