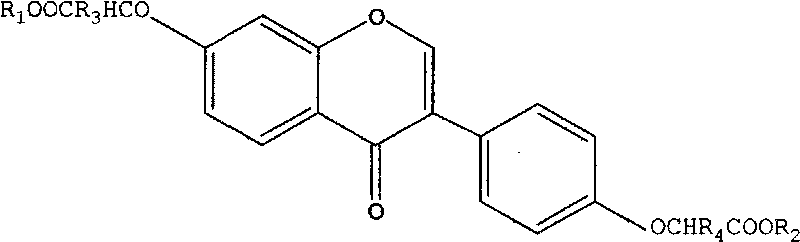
Kudzu-vine root daidzein-7, 4'-dioxo acetic acid compound transfusion formula and preparation method thereof
A technology of dioxoacetic acid and sodium dioxoacetate, which is applied in the field of medicine, can solve problems such as not being widely used, achieve good drug efficacy, facilitate operation, and prevent drug liquid pollution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
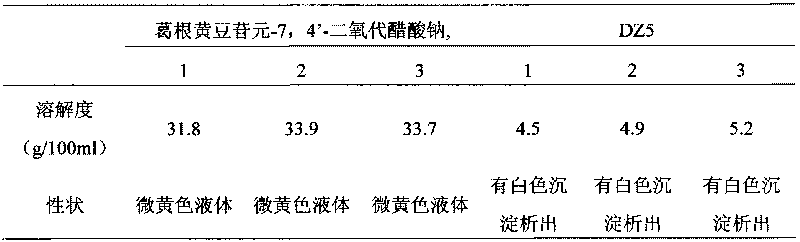
[0024] Example 1: Investigation of Pueraria daidzein-7,4'-sodium dioxoacetate and DZ5 saturated solubility in water
[0025] Take three parts each of excessive daidzein-7,4'-dioxoacetate and DZ5, put them into 10ml plugged centrifuge tubes, add 10ml of distilled water, put the above centrifuge tubes at 25°C, 60r / min in a constant temperature water-bath shaker, after shaking for 24 hours, take it out at the designated time point, centrifuge, absorb an appropriate amount of supernatant, filter it through a 0.45 μm microporous membrane, discard the initial filtrate, and then dilute it with distilled water. The peak area was determined by high performance liquid phase analysis method, and substituted into the standard curve to obtain the concentration. The results are shown in Table 1.
[0026] Table 1 Determination of Saturation Solubility of Pueraria daidzein-7,4'-sodium dioxoacetate and DZ5 in water
[0027]
Embodiment 2
[0028] Embodiment 2: Determination of the range of the pH value of the medicinal solution
[0029] The pH value of human plasma is 7.4, and the pH range of commonly used infusion solutions is 3.2-8.5. In order to determine the pH value range of this product, the pH value range of this product is designed to be 5.0-8.5, and the stability of the liquid medicine is investigated. According to the preparation process, prepare daidzein-7,4'-dioxoacetate sodium, DZ5 solution with a specification of 600mg: 100ml, and adjust the pH to 5.0, 5.5, 6.0, 6.5, 7.0, 7.5, 8.0 and 8.5 after equalization , Sterilize at 121°C for 15 minutes, investigate the stability before and after sterilization, and determine its suitable pH. The results are shown in Table 2.
[0030] Table 2 Sterilization stability investigation results of Pueraria daidzein-7,4'-dioxoacetate sodium under different pH conditions
[0031]
[0032]
[0033] Conclusion: Pueraria daidzein-7,4'-dioxoacetate sodium is stabl...
Embodiment 3
[0034] Embodiment 3: Influence of filling nitrogen or not on infusion stability during filling
[0035]According to the preparation process, prepare daidzein-7,4'-dioxoacetate sodium infusion with specifications of 600mg: 100ml, adjust isotonicity with sodium chloride, adjust pH to 7.5 with sodium hydroxide, divide into two parts, one part Nitrogen gas is filled during filling, and no nitrogen gas is filled when one portion is filled, sterilized at 121°C for 15 minutes, placed under high temperature and accelerated light conditions for 10 days, and the appearance of each trial product is observed. The results are shown in Table 3.
[0036] Influence of Nitrogen Filling on Infusion Stabilizers during Table 3 Filling
[0037]
[0038] Conclusion: Pueraria daidzein-7,4'-dioxoacetate sodium is stable for 10 days under the condition of nitrogen filling, high temperature and accelerated light.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com