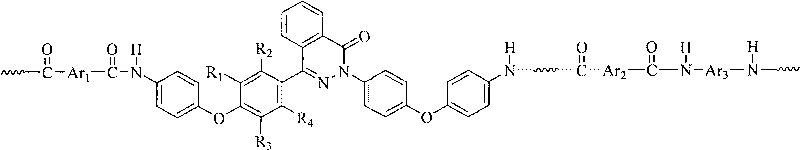
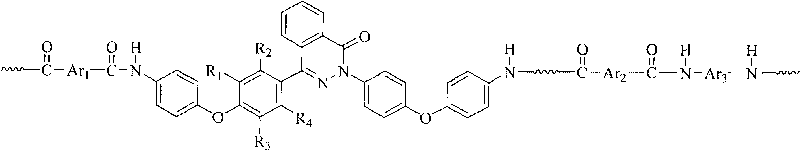
Nomex containing 2,4-bis(4-phenoxyphenyl)chinazolin-1-ketone structure and preparation method thereof
A technology of phenoxyphenyl and phthalazine, which is applied in the field of polymer science, can solve the problems of equipment corrosion environment, difficult processing, high cost, etc., and achieve good mechanical properties, excellent heat resistance stability, and broad application prospects Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] In a three-necked flask equipped with a stirrer, an ice-water bath, and a nitrogen inlet and outlet, sequentially add 4 mmol of 2,4-bis(4-(4-aminophenoxy)phenyl)naphthyridine-1-one, 4.0 mmol p-phenylenediamine, 0.375g of anhydrous lithium chloride, 27ml of N-methylpyrrolidone and an appropriate amount of pyridine, stirred to dissolve them all, then added 8mmol of terephthaloyl chloride while stirring, under nitrogen protection at 0 ℃ reaction, after 10 minutes, the viscosity of the reaction system increased significantly, and there was an obvious rod climbing phenomenon. After reacting for 6 hours, the reaction product was settled in 400ml of ethanol and water (1:1) to obtain a white blocky copolymer, which was filtered and fully washed with hot water, and the polymer was vacuum-dried at 120°C for 24 hours to obtain a poly Aramid resin. The polymer was dissolved in 98% concentrated sulfuric acid, and the intrinsic viscosity was measured with an Ubbelohde viscometer to ...
Embodiment 2
[0041] In a three-necked flask equipped with a stirrer, an ice-water bath, and a nitrogen inlet and outlet, successively add 3 mmol of 2,4-bis(4-(4-aminophenoxy)phenyl)naphthyridine-1-one, 1 mmol of 4,4'-diaminodiphenyl ether, 4.0mmol of p-phenylenediamine, 0.375g of anhydrous lithium chloride, 27ml of N-methylpyrrolidone and an appropriate amount of pyridine, stirred to dissolve them all, and then added 8mmol while stirring Terephthaloyl chloride was reacted at 0°C under the protection of nitrogen, and the system had obvious rod climbing phenomenon, and the viscosity of the reaction system increased significantly after 10 minutes. After reacting for 6 hours, the reaction product was precipitated in 400ml of ethanol and water (1:1) to obtain a white blocky copolymer, which was filtered and washed thoroughly with hot water, and the polymer was vacuum-dried at 120°C for 24 hours to obtain pure Copolyaramid resins. The polymer was dissolved in 98% concentrated sulfuric acid, and...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com