Heparin complex as well as preparation method and application thereof
A complex and heparin technology, which can be used in pharmaceutical formulations, medical preparations with inactive ingredients, and emulsion delivery, etc., can solve the problems of the safety of heparin complexes and the unsatisfactory drug-carrying capacity of stable protein drugs.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0030] The preparation method of the complex formed by grafting heparin onto the poloxamer utilizes the terminal hydroxyl group of the poloxamer and the active group of the carboxyl group or amino group in the heparin molecule. It can be a poloxamer or a poloxamer with a spacer molecule attached to the hydroxyl terminal carboxylation, and then react with the amino group of heparin; it can also be a poloxamer or a poloxamer with a spacer molecule attached to the hydroxyl terminal amino group and then react with the carboxyl group of heparin. Concrete reaction process is as follows:
[0031] a. Carboxylation of poloxamer or poloxamer with inter-arm molecules at the hydroxyl end, followed by reaction with the amino group of heparin
[0032] A certain amount of poloxamer or poloxamer, succinic anhydride, 4-dimethylaminopyridine, and triethylamine that are connected with spacer molecules are placed in dioxane, stirred and reacted overnight, and the resulting reactant The terminal...
Embodiment 1
[0041] Example 1. Synthesis of low molecular weight heparin-poloxamer and its anticoagulation experiment in vitro
[0042] 1-1. Synthesis of low molecular weight heparin-poloxamer-low molecular weight heparin
[0043] Weigh 12 g of Poloxamer 407 (relative molecular weight M=12000, where a=c=101, b=56), and add 2 mmol of succinic anhydride and 2 mmol of 4-dimethylaminopyridine to a round bottom flask and 0.55ml triethylamine, and dissolved in 200ml dioxane, stirred for 24h to obtain poloxamer with a carboxyl group at the end, using it as a reactant, using 1-ethyl-3,3-dimethylammonia Propylcarbodiimide (EDC) / N-hydroxyl diimide (NHS) method, add 10g of low molecular weight heparin sodium, and stir for 24 hours. The reaction product was dialyzed through a dialysis bag for 3 days, and the obtained product was freeze-dried to obtain a solid powder, that is, low molecular weight heparin-poloxamer-low molecular weight heparin.
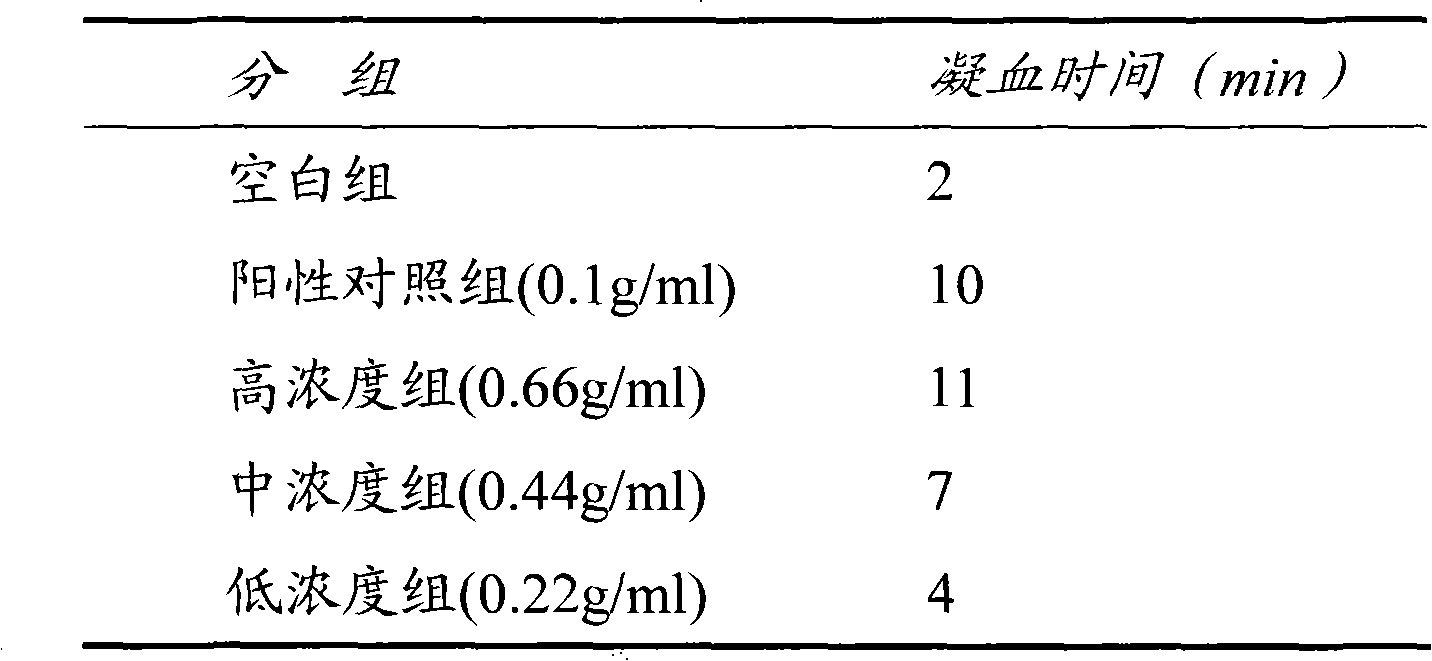
[0044] 1-2. In vitro anticoagulation experiment of low...
Embodiment 2
[0049] Example 2. Preparation of low molecular weight heparin-poloxamer complex micelles
[0050] Take 50 mg of low molecular weight heparin-poloxamer-low molecular weight heparin prepared above, disperse in 20ml of distilled water, and ultrasonically treat at 37°C for 2 minutes to form a micellar suspension, then freeze-dry to make a solid freeze-dried product, According to the in vitro anticoagulant experiment in Example 1, the low molecular weight heparin-poloxamer complex micelles have anticoagulant effect and can be used alone or as a pharmaceutical adjuvant in thrombolytic preparations.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com