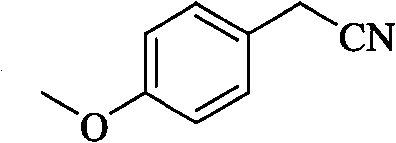
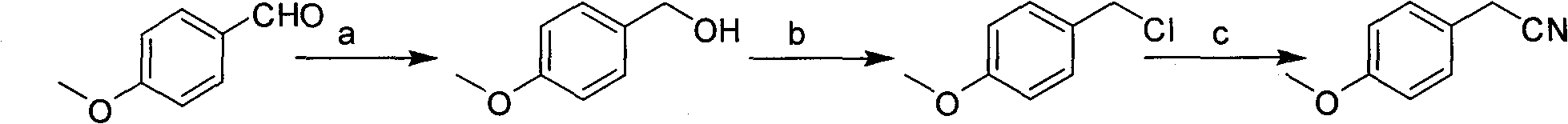Method for preparing para methoxy phenyl acetonitrile
A technology of methoxybenzene acetonitrile and p-methoxybenzene, which is applied in the synthesis of pharmaceutical intermediates and the synthesis of nitriles from aldehydes, can solve problems such as poor operability and yield, and achieve improved total yield and simplified process. , the effect of reducing costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0015] Example 1: the preparation of p-methoxyphenylacetonitrile:
[0016] The preparation of step a p-methoxybenzyl alcohol:
[0017] Take 136g (1mol) of p-methoxybenzaldehyde and add it to a 500ml three-necked flask, control the temperature at 10-40°C, dissolve 19g (0.35mol) of potassium borohydride in 50ml of water, add the potassium borohydride solution dropwise, and use TLC detection end point. After the reaction was completed, 200ml of dichloromethane was added to separate the layers, and the aqueous layer was extracted once with 100ml of dichloromethane, and the organic phases were combined. After the organic phase was dried by adding anhydrous sodium sulfate, it was directly put into the next step reaction, GC>99%.
[0018] Step b: the preparation of p-methoxybenzyl chloride:
[0019] The above organic phase was added into a 1000ml three-neck flask, the reaction temperature was controlled between 20°C and 50°C, 131g (1.1mol) of thionyl chloride was added dropwise, a...
example 2
[0022] Example 2: the preparation of p-methoxyphenylacetonitrile:
[0023] The preparation of step a p-methoxybenzyl alcohol:
[0024] Take 136g (1mol) of p-methoxybenzaldehyde and add it to a 500ml three-necked flask, control the temperature at 10-40°C, take 19g (0.35mol) of potassium borohydride and add it in batches, and control the reaction during the batch addition process. temperature. Then add 200ml of water, continue the reaction, and detect the end point by TLC. 200ml of dichloromethane was added for liquid separation, the aqueous layer was extracted once with 100ml of dichloromethane, and the organic phases were combined. After drying, the solvent was spin-dried to obtain 138 g of product, GC>99%.
[0025] Step b: the preparation of p-methoxybenzyl chloride:
[0026] Add the above product into a 1000ml three-neck flask, add 400ml of dichloromethane to dissolve, control the reaction temperature between 20 and 50°C, take 131g (1.1mol) of thionyl chloride and add it...
example 3
[0029] Example 3: the preparation of p-methoxyphenylacetonitrile:
[0030] Step a: the preparation of p-methoxybenzyl alcohol:
[0031] Take 136g (1mol) of p-methoxybenzaldehyde and add it to a 500ml three-necked flask, control the temperature at 10-40°C, take 13.2g (0.35mol) of sodium borohydride and dissolve it in 50ml of water, add the above solution dropwise, and continue the reaction , TLC detection endpoint. 200ml of dichloromethane was added for liquid separation, the aqueous layer was extracted with 100ml of dichloromethane, and the organic phases were combined. After drying, the solvent was spin-dried to obtain 138 g of product, GC>99%.
[0032] Step b: the preparation of p-methoxybenzyl chloride:
[0033] Add the product from the previous step into a 1000ml three-neck flask, add 400ml of dichloromethane to dissolve, control the reaction temperature between 20 and 50°C, take 131g (1.1mol) of thionyl chloride and drop it into the reaction flask, and control the drip...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com