Niclosamide phosphate ester and pharmaceutically acceptable salt and application thereof
A technology of niclosamide phosphate and niclosamide phosphate, which is applied in the application field of preparing related drugs, can solve the problems of poor compound solubility and poor oral bioavailability, and achieve improved water solubility, improved effect, and improved Effect of Drug Concentration
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
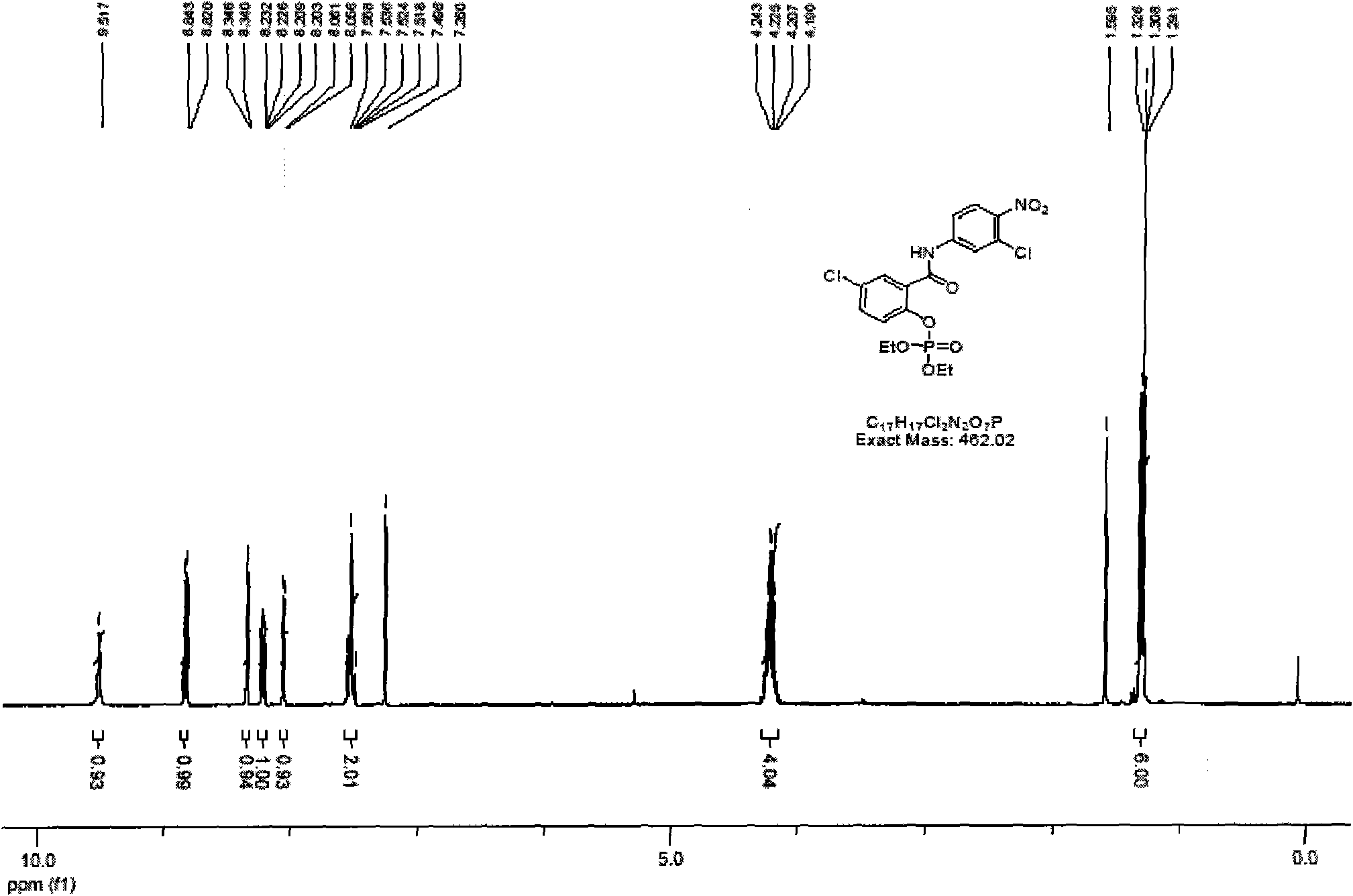
[0025] Synthesis of Compound Niclosamide Phosphate Diethyl Ester 3
[0026] Under nitrogen protection, 8.32g of niclosamide was dissolved in 120mL of anhydrous acetonitrile, and 15.2g of carbon tetrachloride, 5.16g of diisopropylethylamine, and 240mg of N,N-methylaminopyridine were added at -10°C. Add 2.76g of diethyl phosphite dropwise, keep the internal temperature below 0°C, and react for 2 hours. After the reaction is complete, add 0.5M KH2PO4 Quenched, warmed to room temperature and stirred for 1 hour. Most of the organic solvent was evaporated under reduced pressure, extracted by adding 200 mL (X2) ethyl acetate, washed with water, washed with saturated brine, dried by adding anhydrous sodium sulfate, filtered and evaporated under reduced pressure to obtain 11.5 g of light yellow solid quantitatively. 1 H-NMR (400MHz, CDCl 3 )1.33(t, 6H), 4.21(q, 4H), 7.52(m, 2H), 8.06(d, 1H), 8.22(m, 1H), 8.34(d, 1H), 8.23(d, 1H), 9.52(bs, 1H).m / z: 463(M+1)
Embodiment 2
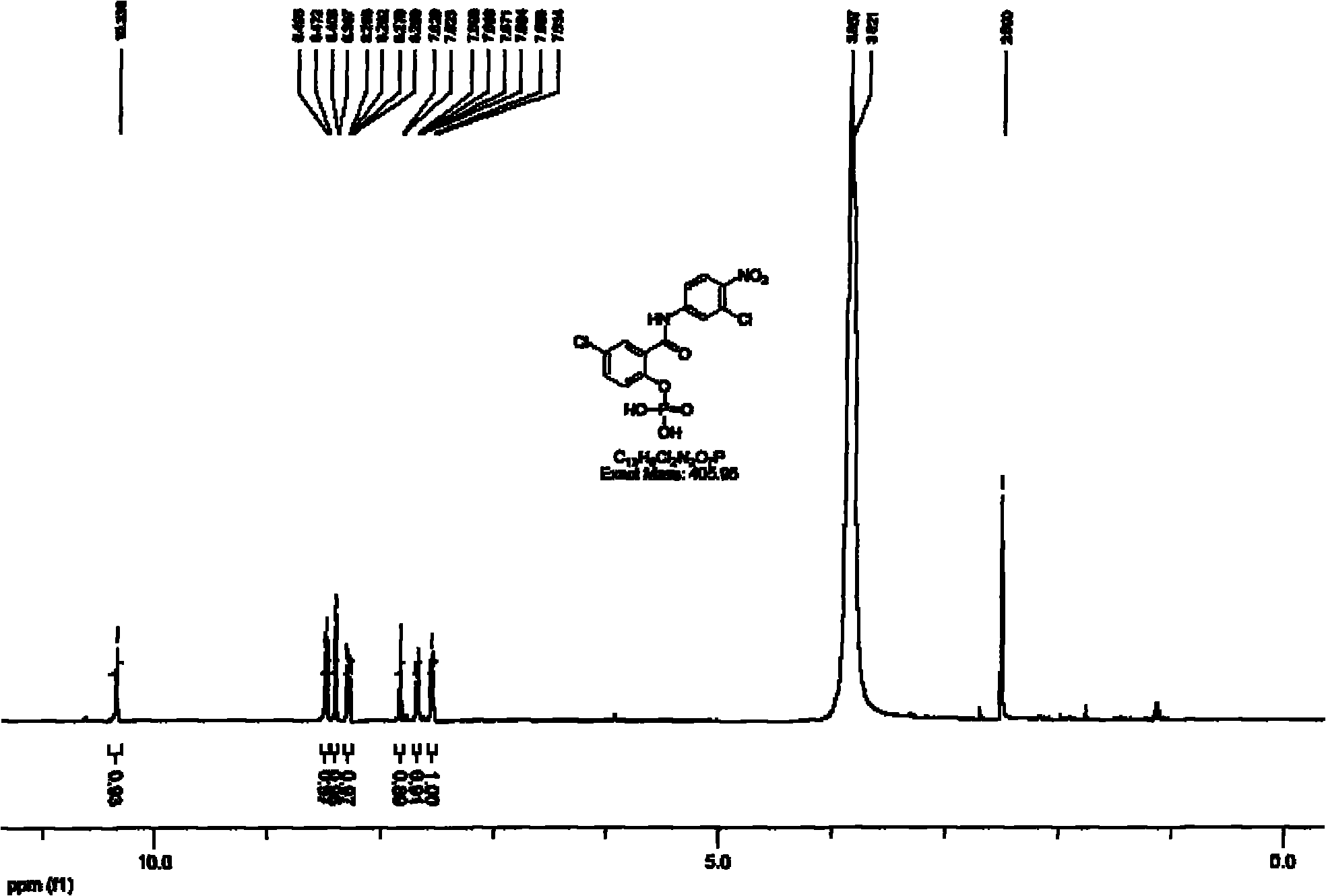
[0028] Synthesis of Compound Niclosamide Monoethyl Phosphate 2a
[0029] Under nitrogen protection, 3.15g of compound 3 was dissolved in 60mL of anhydrous chloroform, 2.1g of trimethylchlorosilane was added dropwise, stirred at room temperature for 8 hours, after the reaction was complete, the solvent was evaporated under reduced pressure, 100mL of methanol was added, and stirred for 30 minutes before Evaporate the solvent under reduced pressure, add 50mL deionized water, adjust the pH to alkaline with ammonia water, add 50mL ethyl acetate to extract twice, separate the water phase, adjust the pH value to 3 with 10% hydrochloric acid, precipitate a large amount of solid, filter, and dry under pressure Finally, 1.3 g of compound 2a was obtained with a yield of 45%. m / z: 435(M+1)
Embodiment 3
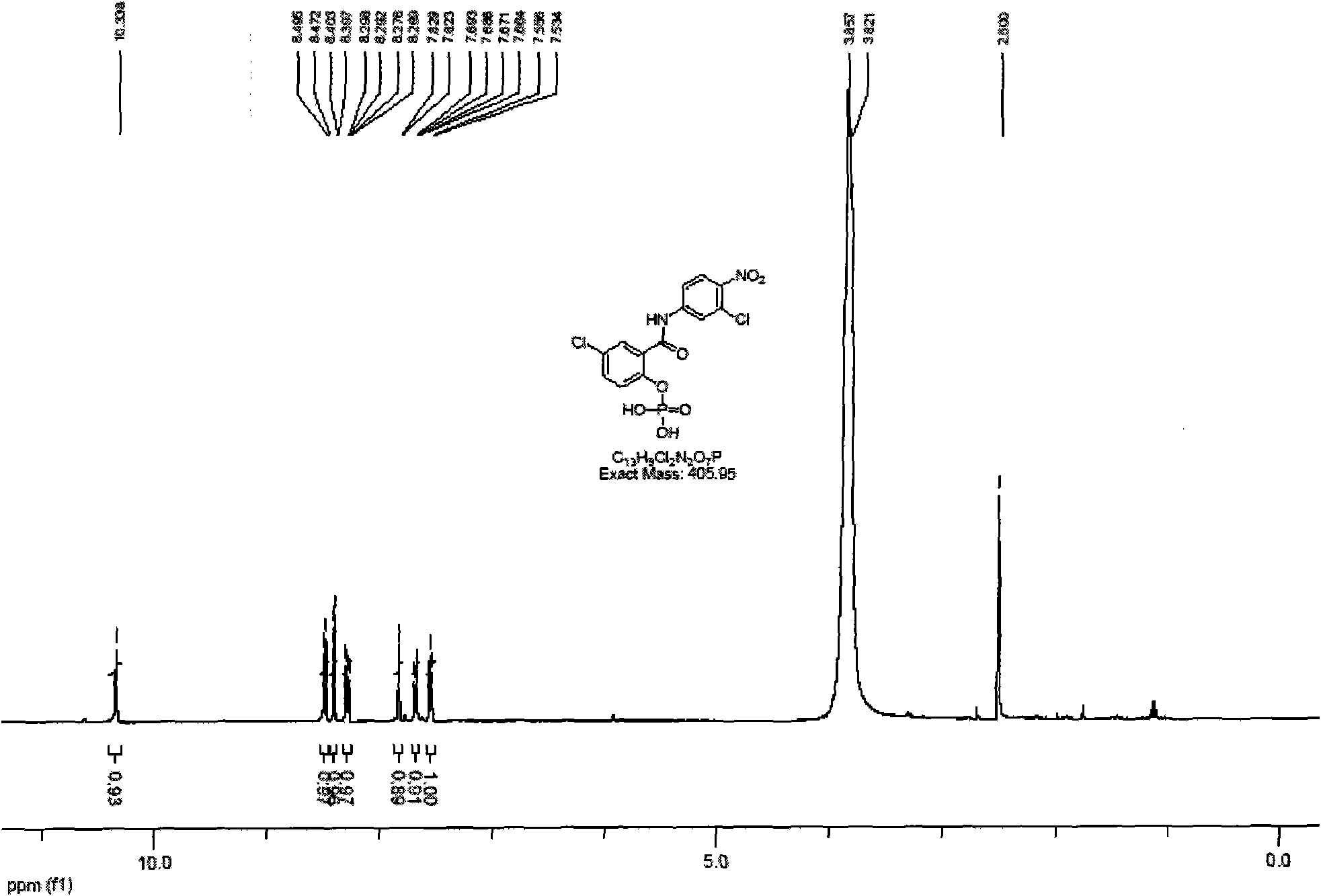
[0031] Synthesis of Compound Niclosamide Phosphate 1
[0032] Under nitrogen protection, 3.15g of compound 3 was dissolved in 60mL of anhydrous chloroform, 5.2g of trimethylchlorosilane was added dropwise, stirred at room temperature for 8 hours, after the reaction was complete, the solvent was evaporated under reduced pressure, 100mL of methanol was added, and stirred for 30 minutes before The solvent was distilled off under reduced pressure to obtain 2.49 g of compound 1 as a white solid with a yield of 90%. 1 H-NMR (400MHz, DMSO) 7.54(d, 1H), 7.67(m, 1H), 7.82(d, 1H), 8.28(m, 1H), 8.40(d, 1H), 8.48(d, 1H). m / z: 407 (M+1).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com