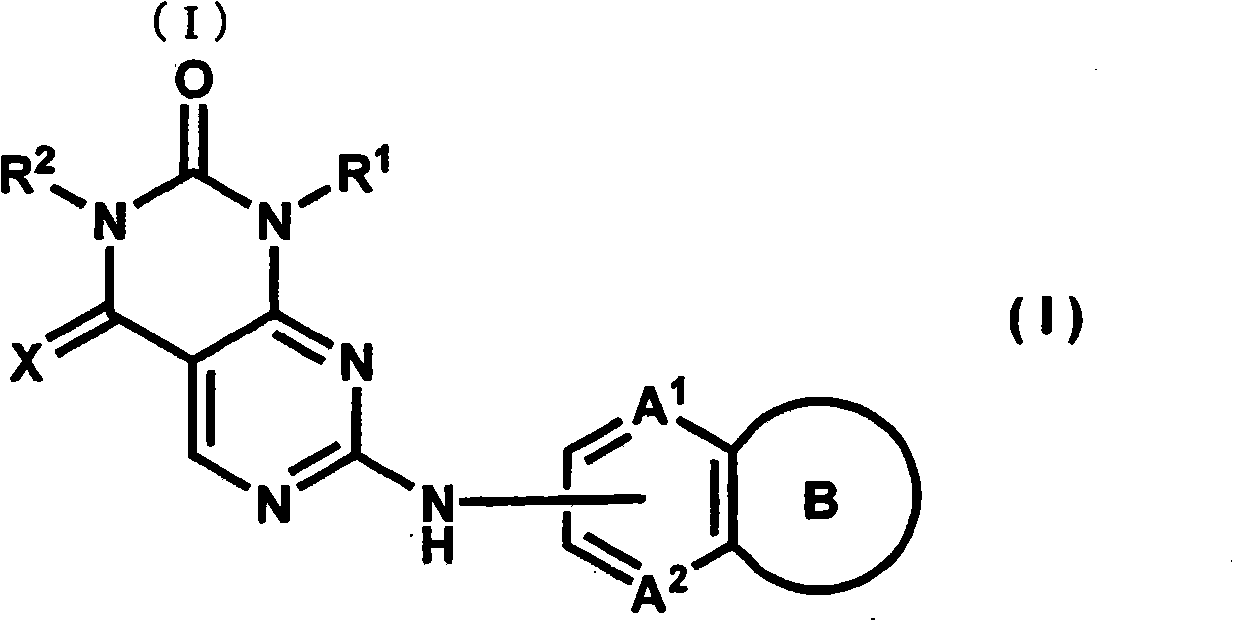
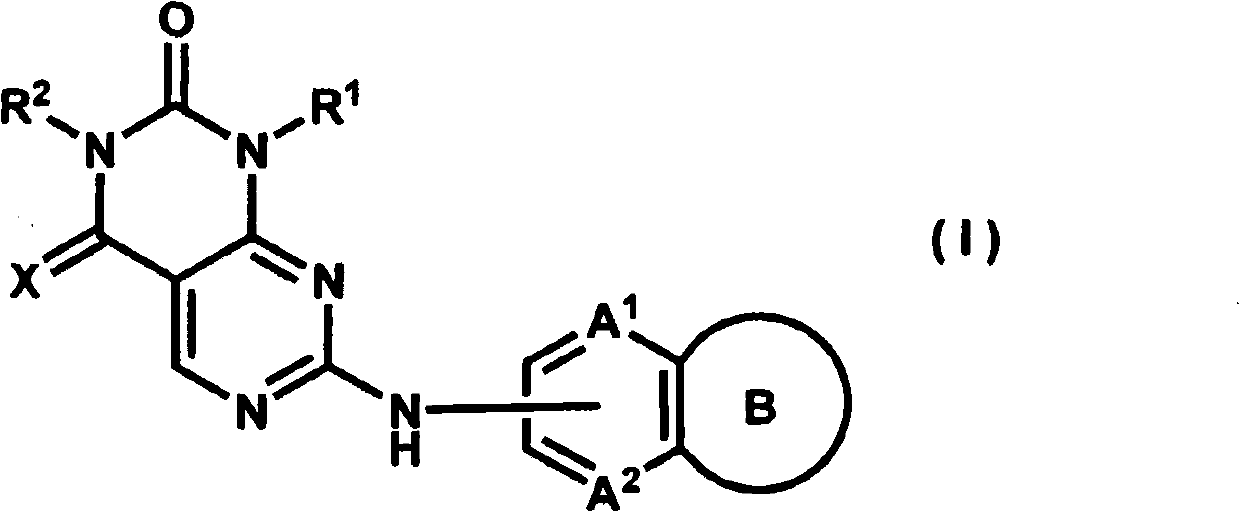
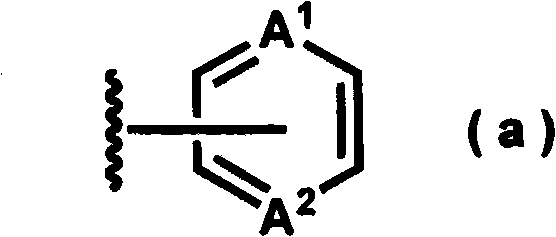
Bicycloaniline derivative
A technology of derivatives and compounds, applied in the field of Wee1 kinase inhibitors, can solve the problems of anticancer agents or reduced radiosensitivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0328] The compound of Example 1 is administered on Day1, 8, and 15
[0329] The tumor proliferation rate was reduced by administration of gemcitabine, but the tumor proliferation rate was further reduced by the combined administration of the compound of the present invention and gemcitabine, and tumor regression was observed in the high-dose combination group.
[0330] As described above, the compounds of the present invention potentiate the effects of other anticancer agents when used in combination with other anticancer agents.
[0331] Pharmacological test 5 (Method for judging drug effect using cells (radiation (X-ray) increase Sensitivity))
[0332] a) Reagents
[0333] Fetal bovine serum (FBS) was purchased from Morgate Company, RPMI1640 medium and 0.25% trypsin EDTA were purchased from Invitrogen Company, Cycle Test Plus DNA kit was purchased from Becton Dickinson Company, and nylon mesh filter was purchased from Millipore Company.
[0334] b) cells
[0335] Hum...
preparation example 1
[0434] 7-Chloro-3-(2,6-dichlorophenyl)-4-imino-3,4-dihydropyrimido Preparation of [4,5-d]pyrimidin-2(1H)-one
[0435]
[0436] To a solution of 3.0 g of 4-amino-2-chloropyrimidine-5-carbonitrile in N,N-dimethylformamide (35 mL) was added 1.12 g of sodium hydride, followed by stirring at room temperature for 5 minutes. 4.38 g of 2,6-dichlorophenylisocyanate was added to the reaction liquid, and stirred at room temperature for 1 hour. Ethyl acetate and 1N aqueous hydrochloric acid solution were added to the reaction solution, and the organic layer was separated. After washing with saturated brine, it was dried over anhydrous magnesium sulfate, and the solvent was distilled off. The precipitated solid was solidified with a methanol-ethyl acetate mixed solvent and collected by filtration to obtain 3.8 g of the title compound as a white solid.
[0437] 1 H-NMR (400MHz, DMSO-d 6 )δ: 9.33 (1H, s), 7.66 (2H, d, J=8.2Hz), 7.53 (1H, t, J=8.2Hz).
[0438] ESI-MS Found: m / z[M+...
preparation example 2
[0440] 7-Chloro-3-(2-chloro-6-methylphenyl)-4-imino-3,4-dihydropyrimidine Preparation of imido[4,5-d]pyrimidin-2(1H)-ones
[0441]
[0442] Except that 298 mg of 2-chloro-6-methylphenyl isocyanate was used instead of 2,6-dichlorophenyl isocyanate used in Preparation Example 1, the same method as Preparation Example 1 was followed to obtain 110 mg of the title compound as a pale yellow solid .
[0443]ESI-MSFound: m / z[M+H]322
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com