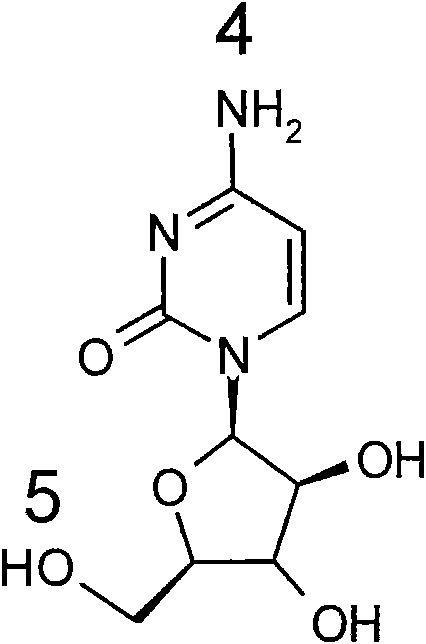
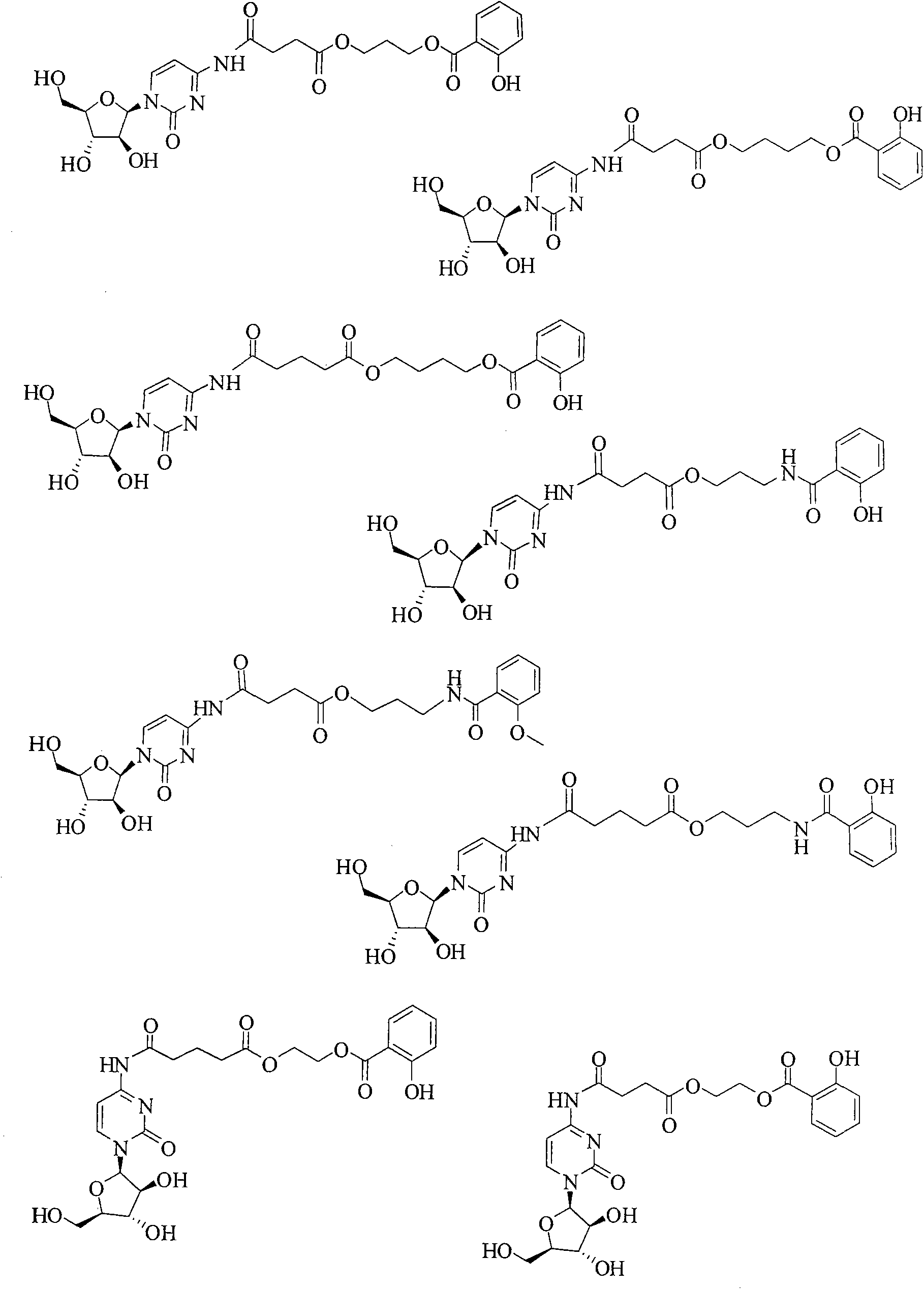
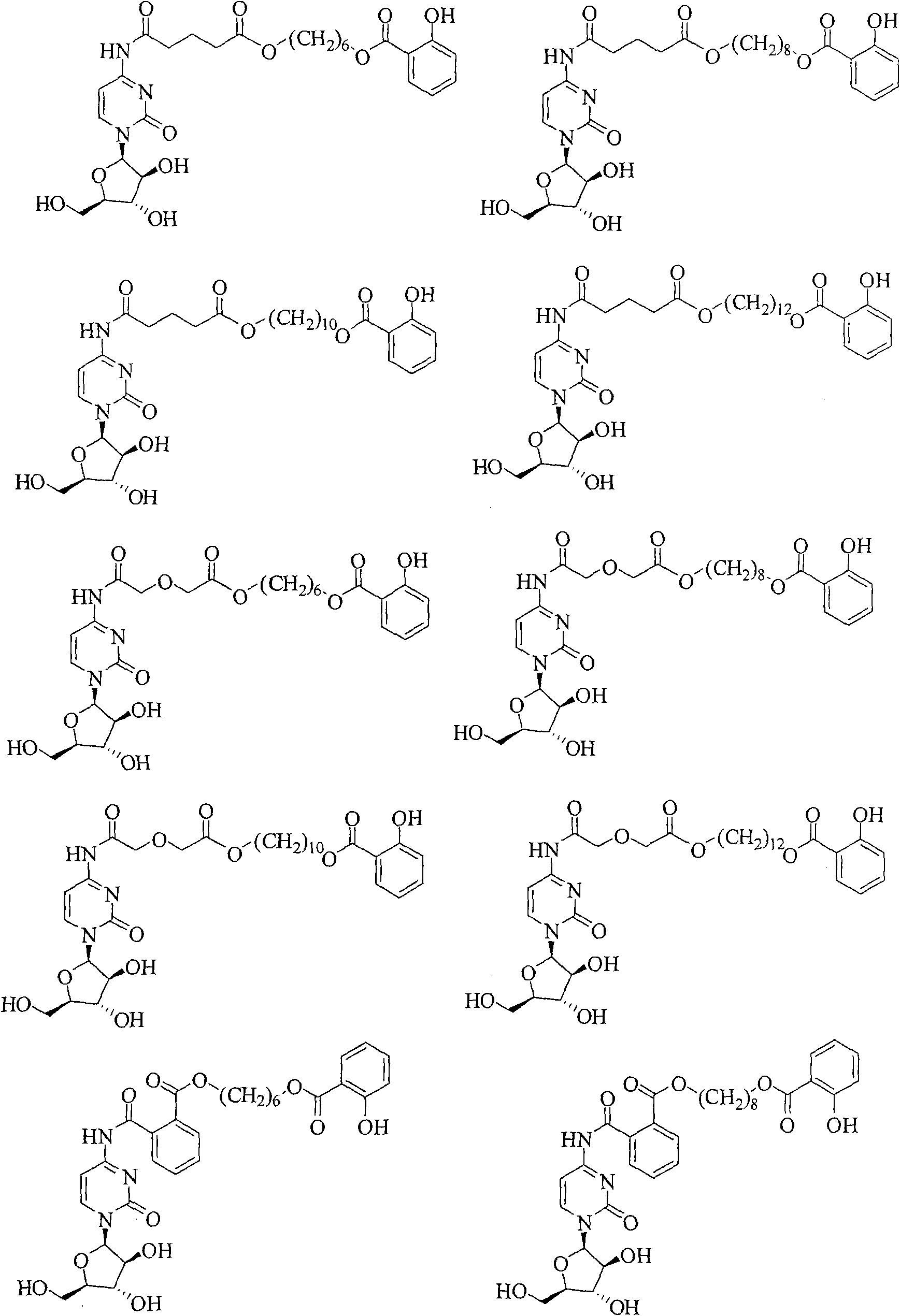
Cytarabine prodrug derivatives and purposes thereof in resisting cancers and tumors
A technology of cytarabine and its derivatives, which is applied in the medical field and can solve problems such as metabolic failure, cytarabine cannot be used to treat liver cancer, and slow activation process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0094] The synthesis routes of some representative cytarabine derivatives of the present invention are listed below, and other cytarabine derivatives in the patent of the present invention are synthesized by the same or similar methods.
[0095] Synthetic route 1:
[0096]
[0097] Synthesis of the first intermediate product B1 (route 1): Dissolve salicylic acid (13.8g, 100mmol) in 40 milliliters of butanediol, then add dropwise 5 drops of concentrated sulfuric acid, reflux reaction for 4 hours, decompression after the end of the reaction Evaporated to dryness, the first intermediate product B1 of gained is directly used in next step reaction
[0098] Synthesis of the second intermediate C1 (Route 1): The first intermediate B1 (2.1 g, 100 mmol) was dissolved in 10 mL of THF, then succinic anhydride (1.0 g, 100 mmol) and DMAP (1.2 g, 100 mmol) were added , stirred at room temperature for 24 hours, filtered the reaction solution, and evaporated the filtrate to dryness under ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com