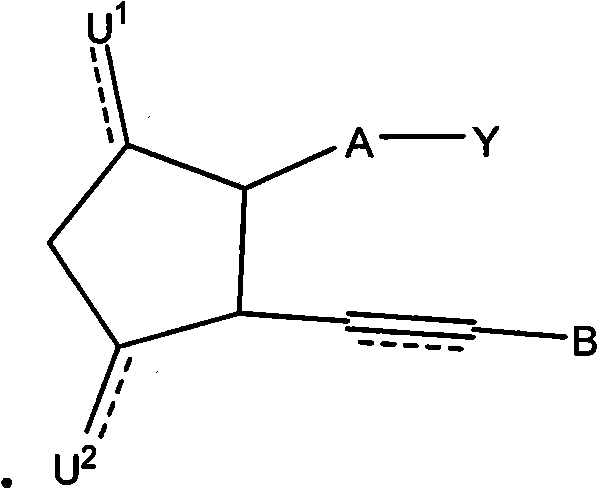
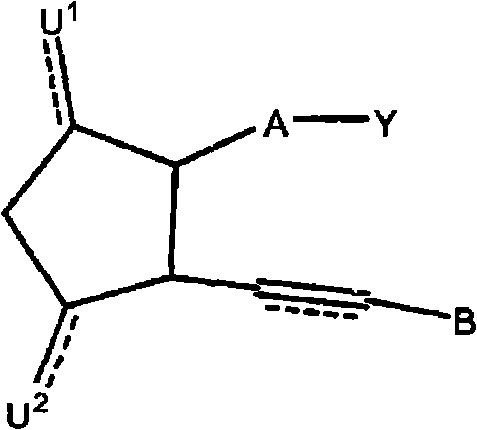
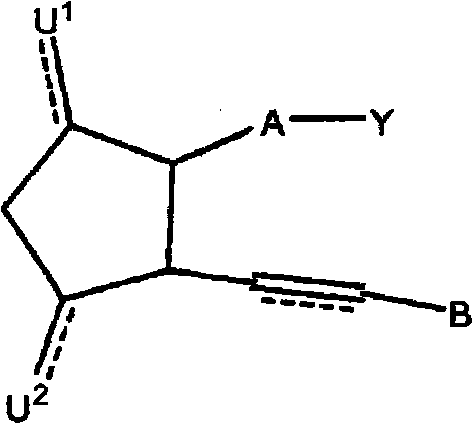
Therapeutic substituted cyclopentanes for reducing intraocular pressure
An intraocular pressure and hydroxycyclopentyl technology, which is applied in the directions of organic active ingredients, preparation of organic compounds, active ingredients of heterocyclic compounds, etc., can solve problems such as obstruction of aqueous humor discharge
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0215] 5-(3-((1R,2R,3R,5R)-5-chloro-2-(3,5-dichlorostyryl)-3-hydroxycyclopentyl)prop-1-enyl)thiophene- 2-Formic acid (8)
[0216] Step 1. Mesylation of 1 to give 2
[0217] Triethylamine (4.2 mL, 30.0 mmol) and methanesulfonyl chloride (1.9 mL, 24.1 mmol) were added sequentially to 1 at 0 °C (see U.S. Provisional Patent Application No. 60 / 805,285, 10.1 g, 19.9 mmol) in CH 2 Cl 2 (100mL) in solution. The reaction mixture was warmed to room temperature and stirred at room temperature for 3 h. Add saturated NaHCO 3 aqueous solution (400mL) and CH 2 Cl 2 (3x400 mL) extracted the mixture. The combined organic extracts were washed with water (200 mL) and brine (200 mL), then dried (MgSO 4 ), filtered and concentrated in vacuo to afford 11.5 g (ca. 98%) of the desired mesylate 2, which was used without further purification.
[0218] Step 2. Conversion of mesylate 2 to chloride 3 and alcohol 4
[0219] Tetrabutylammonium chloride (26.5 g, 95.4 mmol) was added to a solution o...
Embodiment 2
[0233] 5-(3-((1R,2R,3R,5R)-5-chloro-2-(3-chloro-5-(hydroxymethyl)styryl)-3-hydroxycyclopentyl)propyl)- Thiophene-2-carboxylic acid (15a)
[0234] Oxidation in step 1.9 yields 10
[0235] DMSO (32 μL, 0.45 mmol) was added to oxalyl chloride (0.1 mL 2.0 M in CH 2 Cl 2 Solution in, 0.2mmol) in CH 2 Cl 2 (0.3 mL) in solution. After 30 min, alcohol 9 (see U.S. Provisional Patent Application No. 60 / 805,285, 70 mg, 0.17 mmol) was added via syringe in CH 2 Cl 2 (0.54 mL). After 15 min at -78 °C, triethylamine (187 μL, 1.34 mmol) was added and the reaction was allowed to warm to room temperature. After 5 h at room temperature, the reaction mixture was dissolved in saturated NaHCO 3 aqueous solution (10 mL) and CH 2 Cl 2 (20mL). Separate the phases and wash with CH 2 Cl 2 (2x10 mL) to extract the aqueous phase. The combined organic phases were dried (MgSO 4 ), filtered and concentrated in vacuo to afford 69 mg of crude aldehyde 10 which was used without further purificat...
Embodiment 3
[0243] 5-(3-((1R,2R,3R,5R)-5-chloro-2-((E)-2-(5-chloropyridin-3-yl)vinyl)-3-hydroxycyclopentyl) Propyl)-thiophene-2-carboxylic acid (15b)
[0244] Step 1.10 carries out Wittig reaction to obtain 11b
[0245] Following the procedure in step 2 of Example 2, aldehyde 10 (190 mg, 0.46 mmol) and ((5-chloro-3-pyridyl)methyl)triphenylphosphonium chloride (Formulation 2, 100 mg, 0.24 mmol) were converted to 104 mg (84%) alkene 11b (contains about 5% cis-alkene 12b impurity).
[0246] Step 2.11b deprotection gives 13b
[0247] Following the procedure in step 3 of Example 2, THP-ether 11b (104 mg, 0.20 mmol) was converted to 40 mg (46%) of alkene 13b (with about 5% cis alkene 14b impurity).
[0248] Step 3.13b saponification gives 15b
[0249] Following the procedure in Example 2, Step 4, ester 13b (10 mg, 0.023 mmol) was converted to 3 mg (31%) of the title compound (containing about 5% cis-olefin 16b impurity).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com