Inhibitors of receptor tyrosine kinases and methods of use thereof
A technology of tyrosine kinase and tyrosine kinase cell, applied in receptor tyrosine kinase inhibitor and its application field, can solve the problem of incomprehensible capacitative mutation and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 22-25
[0295] Moieties of the invention can be screened for RTK inhibitory activity using any of the assays described herein and those well known in the art. For example, assays that measure receptor internalization, receptor autophosphorylation, and / or kinase signaling can be used to identify components that prevent activation of a target RTK (eg, a Kit receptor). can be obtained by using standard methods known in the art, for example by using phosphoELISA TM method (available at Invitrogen) to determine the phosphorylation status of RTKs or downstream molecules, enabling the screening of new inhibitor components. The phosphorylation state of the receptor (such as the Kit receptor) can be obtained using a commercially available kit, such as C-Kit[pY823]ELISA KIT, HU (BioSource TM ; CatalogNumber-KHO0401); c-KIT[TOTAL]ELISA KIT, HU (BioSource TM ; Catalog Number-KHO0391), to be determined. Antibodies, small molecules and other components of the invention can be screened using such...
Embodiment 1
[0359] Example 1: Expression, purification and crystallization of SCF and Kit
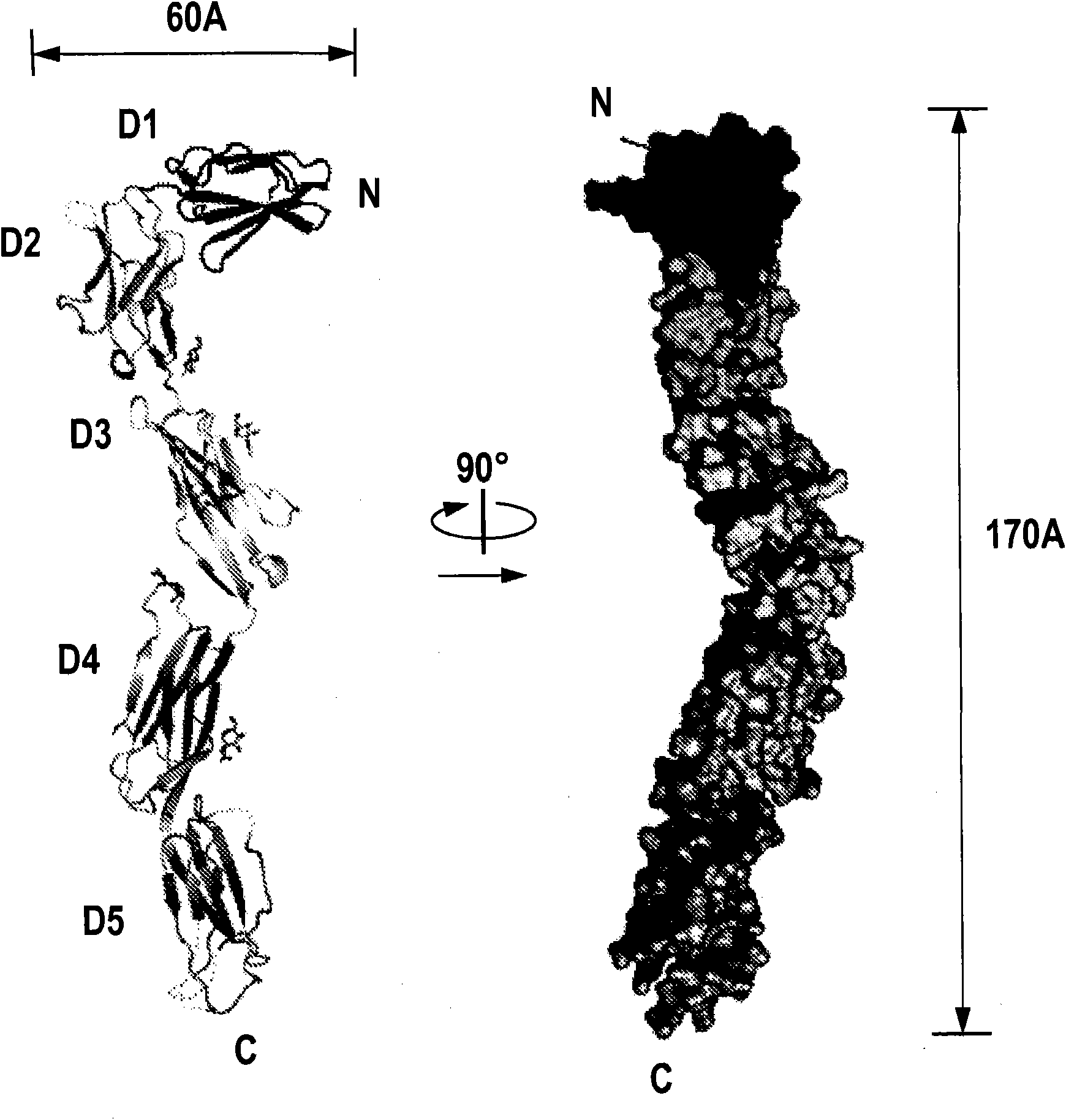
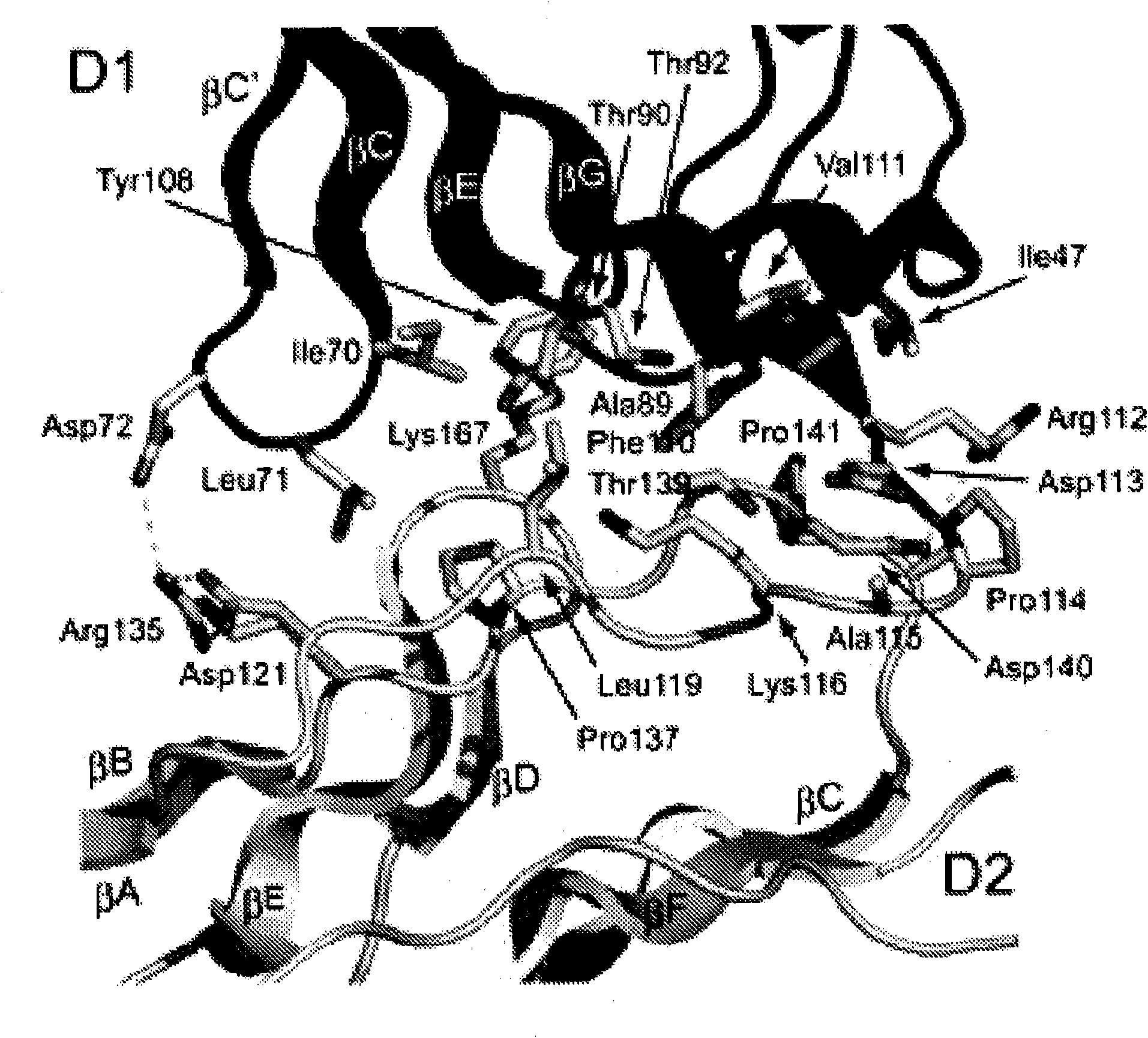
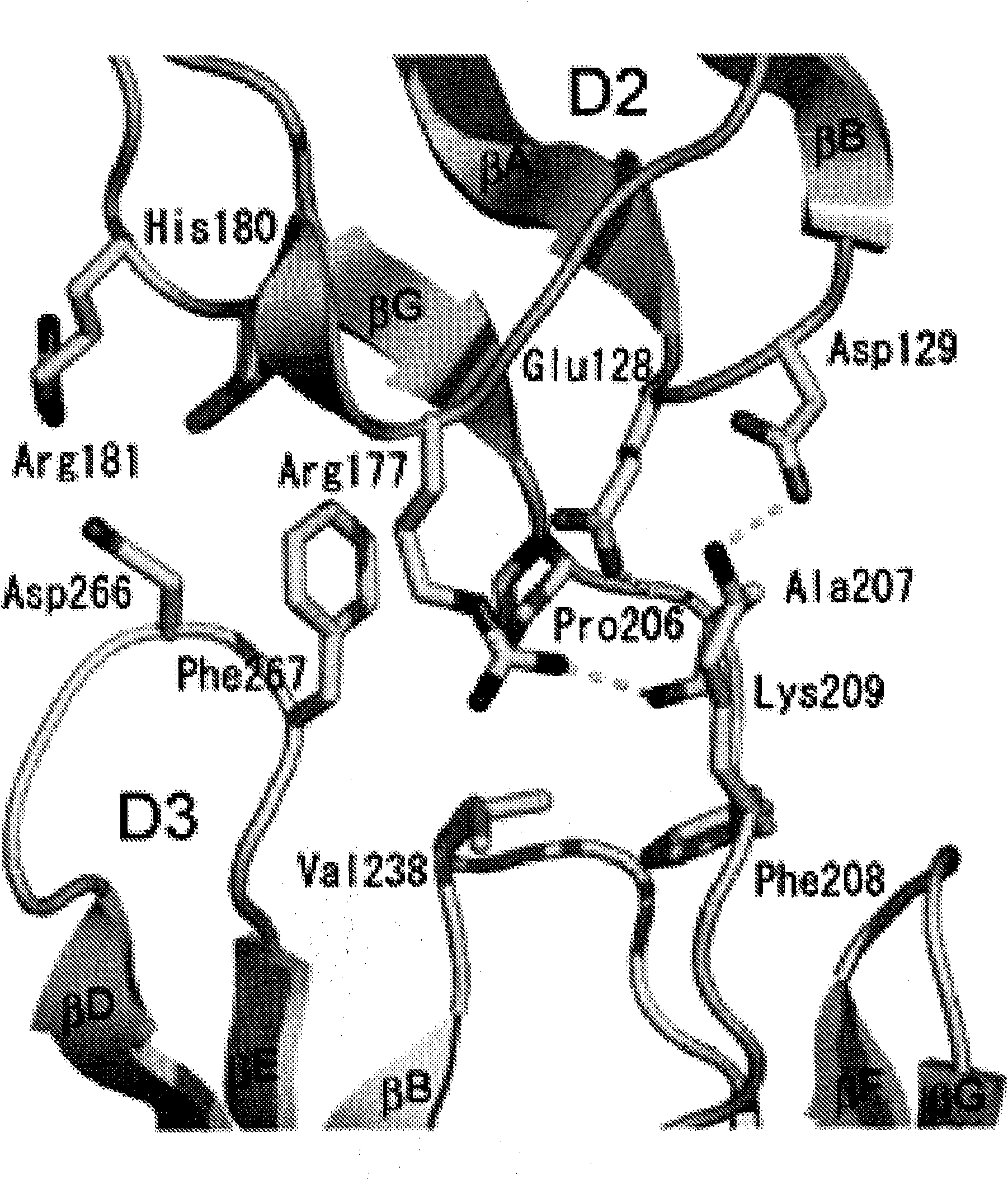
[0360] The complete ectodomain of Kit consisting of five Ig-like domains called D1, D2, D3, D4 and D5 was expressed in insect cells using the baculovirus expression system. Purified Kit ectodomain monomers or SCF-induced Kit ectodomain homodimers (SCF-Kit2:2 complexes) were each subjected to large-scale screening for crystal growth and optimization, followed by determination of their crystal structures.
[0361] Protein Expression and Purification
[0362] The soluble Kit ectodomain (amino acids 1-519) containing a polyhistidine tag at the C-terminus was expressed in insect cells (Sf9) using the baculovirus expression system. The Kit ectodomain was purified by Ni chelation followed by size exclusion chromatography (Superdex 200, GE Healthcare). After partial deglycosylation using endoglycosidase F1, the extracellular domain was further purified by anion exchange chromatography (MonoQ, GE Health...
Embodiment 2
[0368] Example 2: Structure Determination
[0369] By using multiple isomorphic substitution with anomalous scattering (MIRAS) and by multiwavelength anomalous scattering (MAD) at 3.0 The resolution was calculated for the experimental phase (Table 1A). The resulting electron density map shows a continuous electron density of the β-sandwich structure and a clear solvent-protein boundary. The molecular model of the monomeric Kit ectodomain was artificially constructed into the experimental electron density map. The structure was optimized to 3.0 using a raw data set of 25.4% crystalline R-factor and 29.6% free R-factor resolution (Table 1B). SCF-Kit was resolved by molecular replacement using the monomeric form and SCF structure described in this report (Zhang et al. (2000) Proc Natl Acad Sci US A 97:7732-7737; available code: 1EXZ obtained from the Protein Data Bank) as search models Structure of the 2:2 complex. The structure was optimized to 3.5 using the original data...
PUM
| Property | Measurement | Unit |
|---|---|---|
| surface area | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com