Application of 2-phenylphenol and derivatives thereof to medicine for treating epilepsia
A technology of biphenol and derivatives, applied in the field of medicine, to achieve the effects of strong affinity, excitotoxicity protection, and brain protection
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
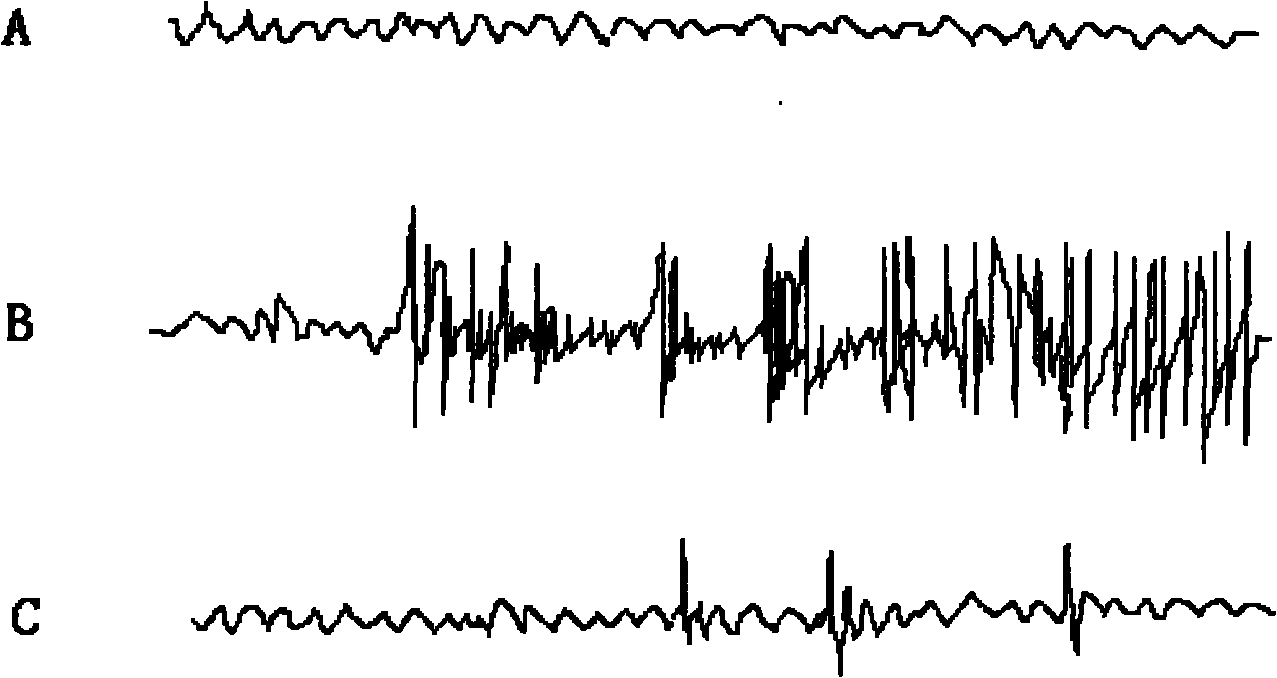
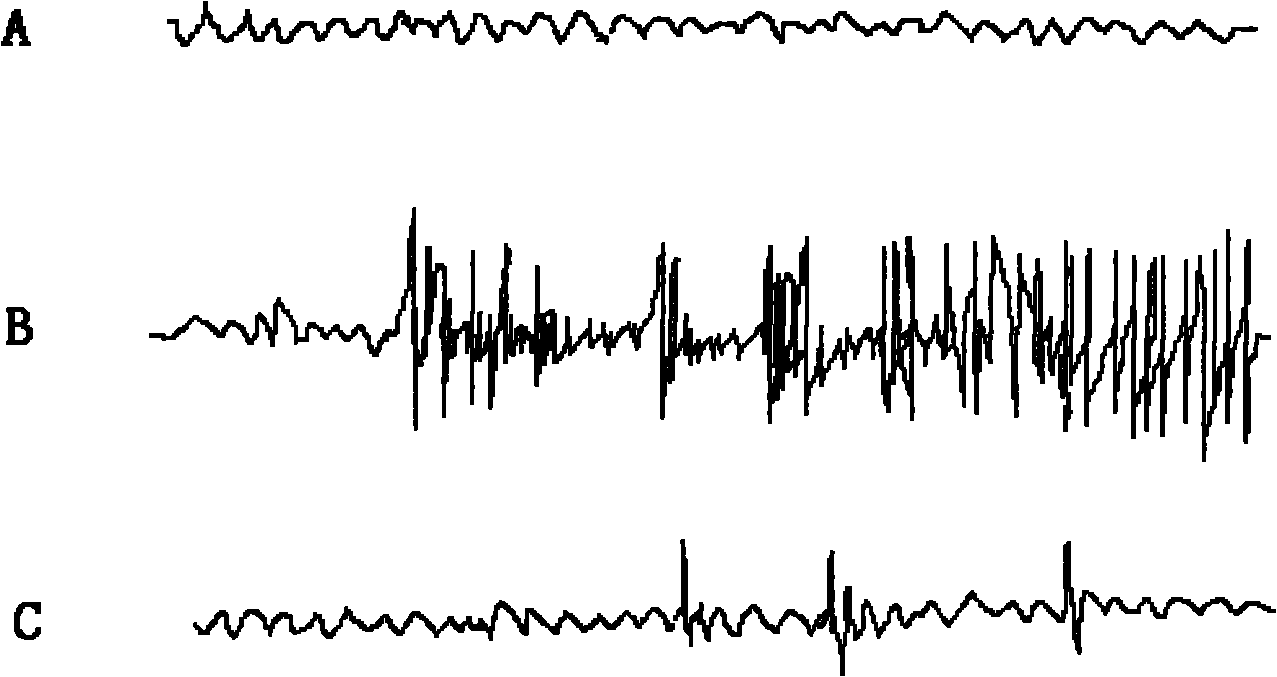
[0027] Effects of 2,2',6,6'-tetraisopropyl-4,4'-bisphenol on cortical EEG of pentylenetetrazol epilepsy model rats during seizures Materials: Male SD rats, body weight 250~ 300g, purchased from the Experimental Animal Center of Fourth Military Medical University. Pentetrazol (Sigma, USA).
[0028] Main instruments: multi-channel physiological recorder, special electrodes, dental bench drill, general-purpose electroencephalograph.
[0029] method:
[0030] (1) Preparation of pentylenetetrazol epilepsy model
[0031] 35mg / kg pentetrazol was injected intraperitoneally to induce seizures.
[0032] (2) Cortical EEG recordings of pentylenetetrazol epilepsy model rats during seizures
[0033] Rats were first anesthetized with amobarbital. The scalp was incised to expose the skull, and a small hole was drilled with a dental electric drill at the left and right sides of the junction of the coronal suture and sagittal suture 3 mm away, electrodes were implanted, and the scalp was s...
Embodiment 2
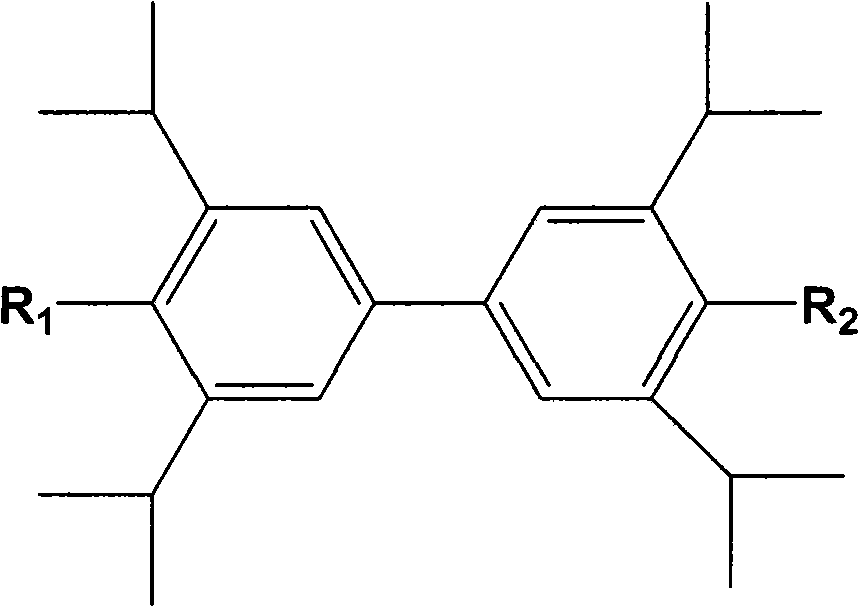
[0042] Other biphenols or derivatives thereof described in the present invention also have the same therapeutic effect as 2,2',6,6'-tetraisopropyl-4,4'-biphenols described in Example 1.
Embodiment 3
[0043] Embodiment 3: pharmaceutical composition
[0044] 2,2',6,6'-tetraisopropyl-4,4'-diphenol passed through 100 mesh sieve for later use; 2,2',6,6'-tetraisopropyl-4,4'- Diphenol 80.0g, microcrystalline cellulose 45.0g, croscarmellose sodium 5.0g, mixed evenly, using aqueous solution as wetting agent to make soft material, granulated with 30 mesh screen, ventilated and dried at 55°C, and prepared granules, set aside; add 0.5 g of micropowder silica gel, 1.0 g of magnesium stearate, mix well, press to obtain about 1000 round tablets, and obtain compressed tablets.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com