Adefovir dipivoxil compound and novel preparation method thereof
A technology of adefovir dipivoxil and compounds, which is applied in the fields of compounds, chemical instruments and methods, organic chemistry, etc. of Group 5/15 elements of the periodic table, and can solve the problems of difficult large-scale production, difficult purification, and adefovir dipivoxil. Problems such as low purity
Inactive Publication Date: 2010-08-25
HAINAN YONGTIAN PHARMA INST
View PDF2 Cites 5 Cited by
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
The adefovir dipivoxil obtained by the above-mentioned patented method has low purity, is not easy to purify, has high production cost, and is not easy to produce on a large scale
Method used
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
View moreImage
Smart Image Click on the blue labels to locate them in the text.
Smart ImageViewing Examples
Examples
Experimental program
Comparison scheme
Effect test
Embodiment 1
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More PUM
 Login to View More
Login to View More Abstract
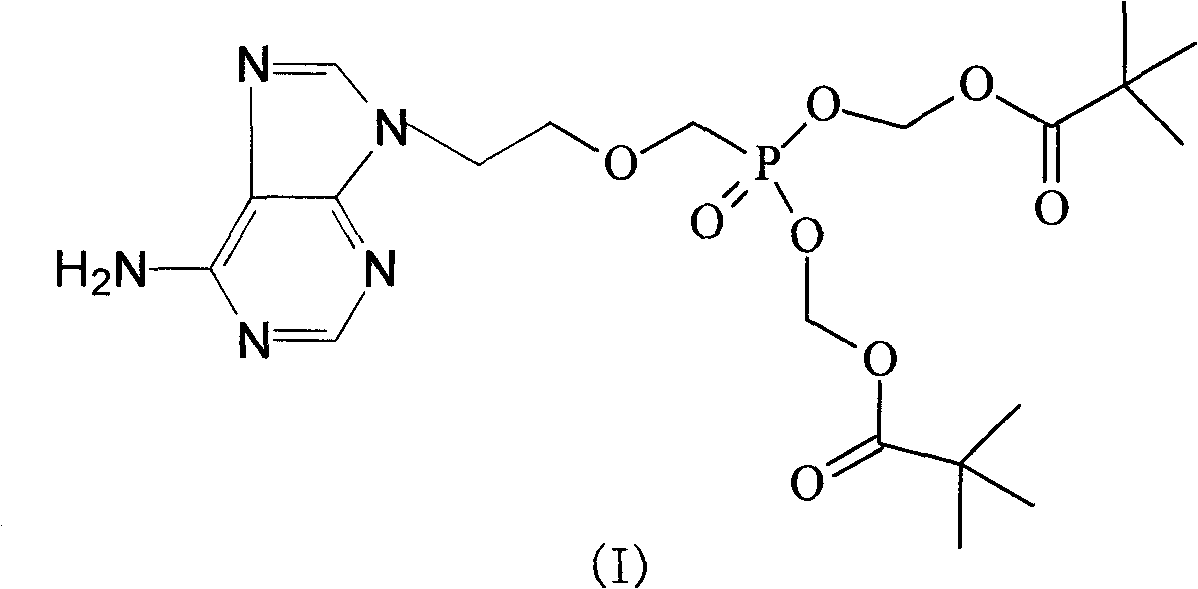
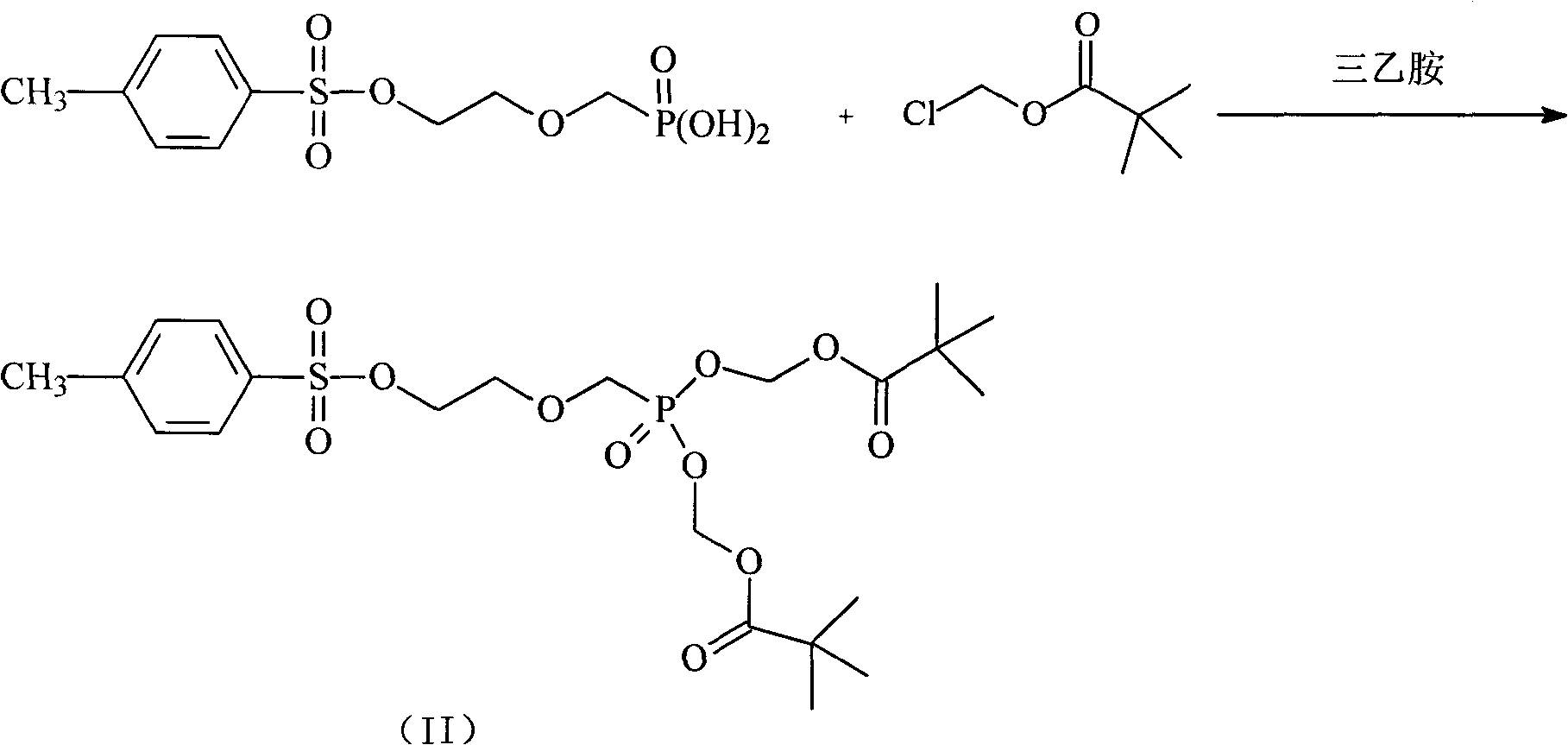
The invention aims to provide an adefovir dipivoxil compound and a novel preparation method thereof; the method specifically comprises the following steps that: (2-p-toluenesulfonyloxy ethoxy) diethyl methylphosphonate is hydrolyzed at low temperature, to prepare (2-p-toluenesulfonyloxy ethoxy) methyl phosphonic acid; chloro methyl pivalate is added in to react and prepare an intermediate body (II) under the action of triethylamine; and adenine and the intermediate body (II) react to prepare adefovir dipivoxil under the catalysis of anhydrous potassium carbonate. The invention reduces the introduction of impurities, improves the purity, and lays foundation for industrial production.
Description
technical field The invention relates to an adefovir dipivoxil compound, belonging to the technical field of medicine. Background technique Adefovir dipivoxil, chemical name: 9-[2-[bis(pivaloyloxymethoxy)phosphorylmethoxy]ethyl]adenine, molecular formula C 20 h 32 N 5 o 8 P, molecular weight 501.47, its structural formula is: Adefovir is an acyclic nucleoside analog of adenosine monophosphate, which is phosphorylated into an active metabolite, adefovir diphosphate, under the action of cellular kinases. Adefovir diphosphate inhibits HBV DNA polymerase (reverse transcriptase) in the following two ways; one is to compete with the natural substrate deoxyadenosine triphosphate, and the other is to cause DNA chain elongation and termination after being integrated into viral DNA. The inhibition constant (Ki) of adefovir diphosphate to HBV DNA polymerase is 0.1 μM, but the inhibitory effect on human DNA polymerase α and γ is weak, with Ki values of 1.18 μM and 0.97 μM, respe...
Claims
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More Application Information
Patent Timeline
 Login to View More
Login to View More Patent Type & Authority Applications(China)
IPC IPC(8): C07F9/6561
Inventor 陶灵刚
Owner HAINAN YONGTIAN PHARMA INST
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com