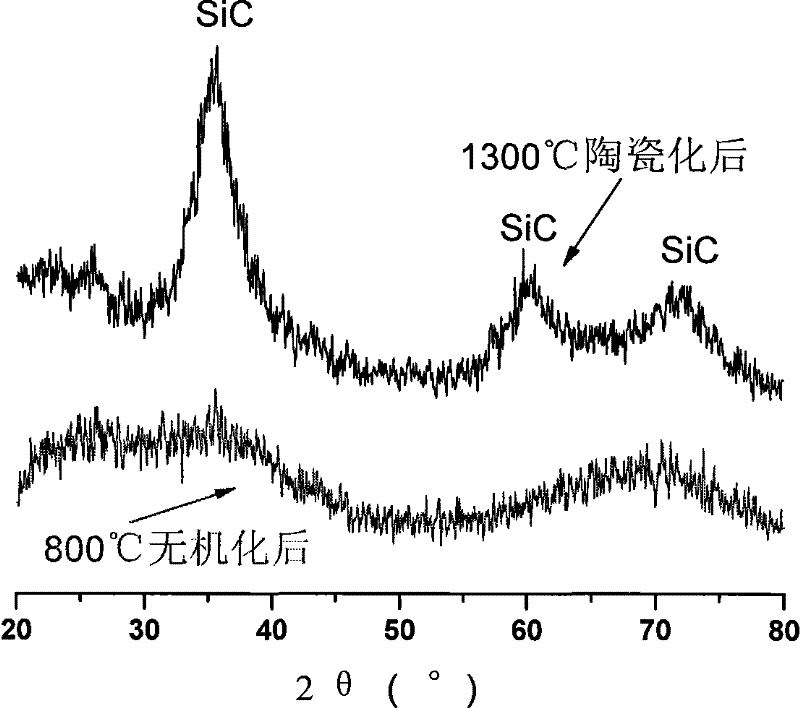
Manufacturing method of SiC-SiBCN nucleated glass
A kind of glass-ceramic and a technology of manufacturing method, which are applied in the field of manufacturing SiC-SiBCN glass-ceramic
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
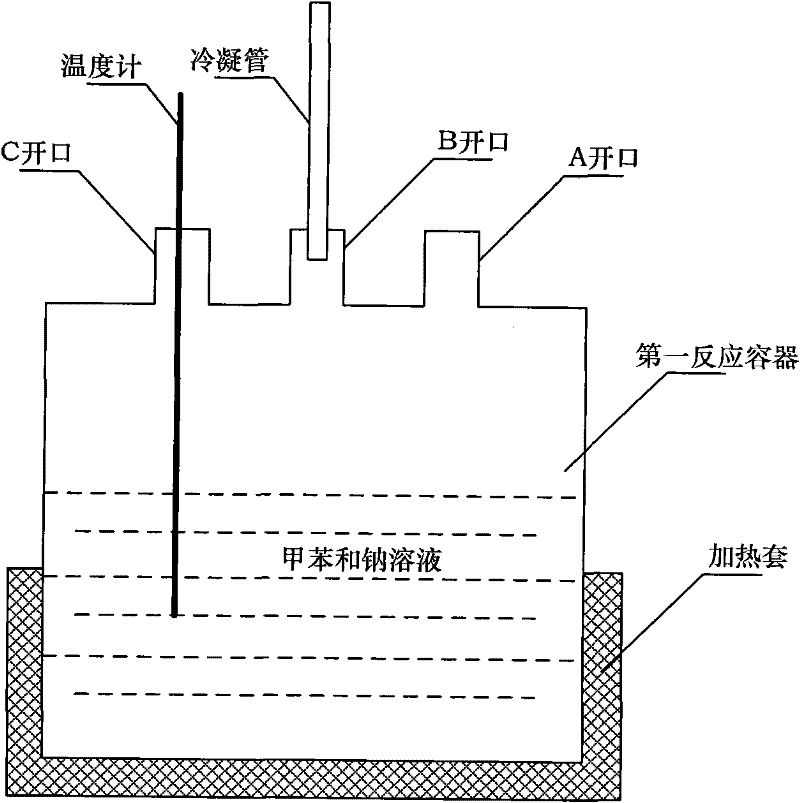
[0057] Step 1: Prepare anhydrous toluene
[0058] Toluene C 6 H 6 The sodium and Na are put into the first reaction vessel through the opening A, reflux at 110℃ (measured by the thermometer in real time) to remove trace water in toluene, and then distilled at 112℃ (measured by the thermometer in real time) Obtain anhydrous toluene; add 5g of sodium to 100ml of toluene;
[0059] Step 2: Preparation of PCS-toluene solution
[0060] Dissolve polycarbosilane (PCS) in the anhydrous toluene prepared in step 1 to form a PCS-toluene solution; add 1g of polycarbosilane to 1ml of anhydrous toluene;
[0061] Step 3: Prepare PBS-toluene solution
[0062] Dissolve polyborosilazane (PBS) in the anhydrous toluene prepared in step 1 to form a PBS-toluene solution; add 1ml of polyborosilazane (PBS) to 1ml of anhydrous toluene;
[0063] Step 4: Precursor polymer alloy
[0064] The PCS-toluene solution prepared in step 2 and the PBS-toluene solution prepared in step 3 were mixed at a magnetic stirring spee...
Embodiment 2
[0074] Step 1: Prepare anhydrous toluene
[0075] Toluene C 6 H 6 And sodium Na is put into the first reaction vessel through opening A, refluxed at 108℃ (measured by thermometer in real time) to remove trace water in toluene, and then distilled at 112℃ (measured by thermometer in real time) Obtain anhydrous toluene; add 3g of sodium to 100ml of toluene;
[0076] Step 2: Preparation of PCS-toluene solution
[0077] Dissolve polycarbosilane (PCS) in the anhydrous toluene prepared in step 1 to form a PCS-toluene solution; add 0.5 g of polycarbosilane to 1 ml of anhydrous toluene;
[0078] Step 3: Prepare PBS-toluene solution
[0079] Dissolve polyborosilazane (PBS) in the anhydrous toluene prepared in step 1 to form a PBS-toluene solution; add 0.5 ml of polyborosilazane (PBS) to 1 ml of anhydrous toluene;
[0080] Step 4: Precursor polymer alloy
[0081] The PCS-toluene solution prepared in step 2 and the PBS-toluene solution prepared in step 3 were mixed at a magnetic stirring speed of 60...
Embodiment 3
[0089] Step 1: Prepare anhydrous toluene
[0090] Toluene C 6 H 6 And sodium Na is put into the first reaction vessel through opening A, and refluxed at 105℃ (measured by thermometer in real time) to remove trace water in toluene, and then distilled at 110℃ (measured by thermometer in real time) Obtain anhydrous toluene; add 1g of sodium to 100ml of toluene;
[0091] Step 2: Preparation of PCS-toluene solution
[0092] Dissolve polycarbosilane (PCS) in the anhydrous toluene prepared in step 1 to form a PCS-toluene solution; add 0.1 g of polycarbosilane to 1 ml of anhydrous toluene;
[0093] Step 3: Prepare PBS-toluene solution
[0094] Dissolve polyborosilazane (PBS) in the anhydrous toluene prepared in step 1 to form a PBS-toluene solution; add 0.1 ml of polyborosilazane (PBS) to 1 ml of anhydrous toluene;
[0095] Step 4: Precursor polymer alloy
[0096] The PCS-toluene solution prepared in step 2 and the PBS-toluene solution prepared in step 3 were mixed at a magnetic stirring speed o...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com