Beta-amino acid esters having optical activity and containing benzothiazole groups and synthetic method and application thereof
A benzothiazole-based, optically active technology applied in the field of benzothiazole-containing beta-amino acid ester compounds
Inactive Publication Date: 2010-09-01
GUIZHOU UNIV
View PDF3 Cites 18 Cited by
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
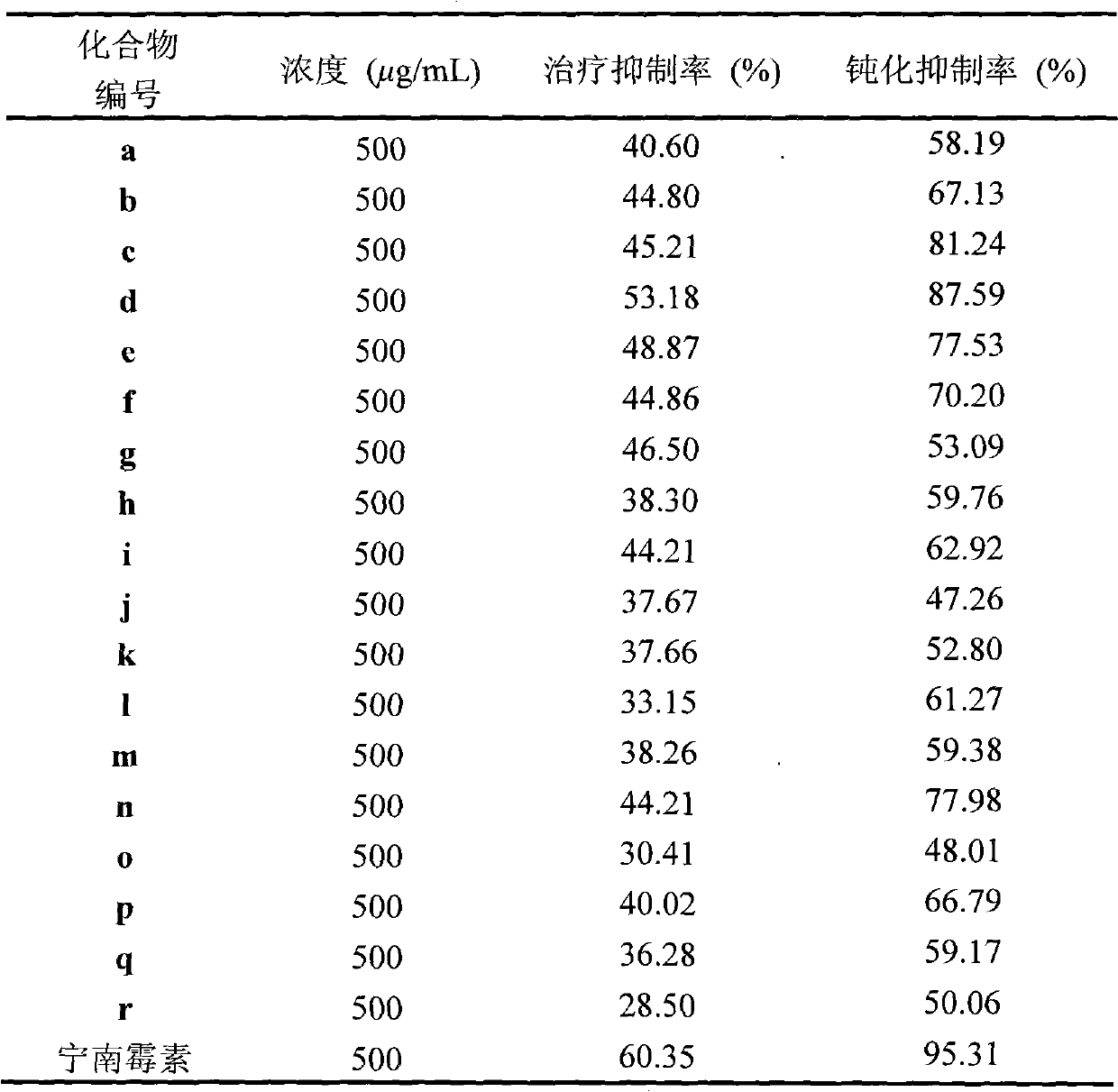
But so far there is no research about the Mannich reaction catalyzed by benzothiazole imine and malonate with cinchonaline thiourea organocatalyst. Therefore, it is of great significance to study the synthesis of β-amino acid compounds containing benzothiazole groups by organocatalytic Mannich reaction
Method used
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
View moreImage
Smart Image Click on the blue labels to locate them in the text.
Smart ImageViewing Examples
Examples
Experimental program
Comparison scheme
Effect test
Embodiment 1
Embodiment 2
Embodiment 3
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More PUM
 Login to View More
Login to View More Abstract
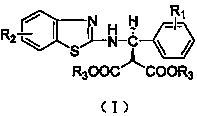
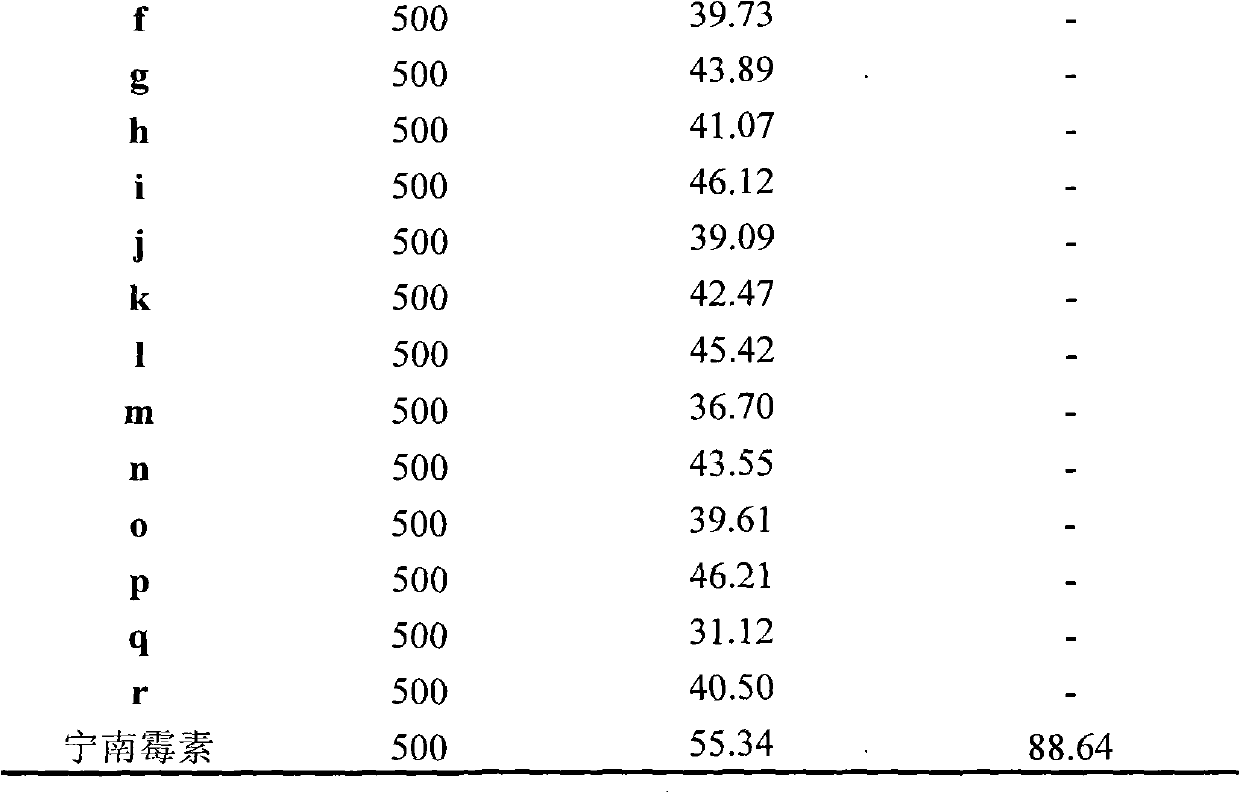
The invention discloses a synthetic method and bioactivity of beta-amino acid esters which have optical activity and contain benzothiazole groups. The beta-amino acid esters which have optical activity and contain the benzothiazole groups have the structure shown in the general formula (I), and in the formula, R1 is a group of hydrogen, p-chloro, o-chloro, p-fluoro, o-fluoro, p-methyl, o-methoxy and the like; R2 is a group of 4-methyl, 6-methoxy and the like; and R3 is a group of methyl, ethyl, propyl, isopropyl and the like. The invention introduces Mannich reaction which uses organic catalyst of cinchona alkaloid thiourea to catalyze benzothiazole imine and malonate, the product is obtained by one-step synthesis, and the reaction yield and the selectivity to enantiomer are both high. Compounds c, d and e in the inveniton have high therapeutic and passivation inhibiting effects towards tobacco mosaic virus (TMV) and cucumber mosaic virus (CMV) and show good anti-plant virus activity.
Description
technical field The invention relates to an optically active β-amino acid ester compound containing a benzothiazole group with an anti-plant virus effect and a preparation method thereof. Background technique Thiazole compounds have broad-spectrum biological activities and can be used in the research of pesticides and medicines. Since Merck company successfully developed thiabendazole (Triabendazole) in 1962, the research on the biological activity of thiazole compounds has caused people's great interest, and has successfully developed a lot of thiazole pesticide varieties (Fitzjohn, S.; Robinson, M.P. Bezoxazole and benzothiazole Derivatives [P]. WO 9406783, 1994-03-31. Naka, I.P.; Matsuno, H., Inami, S. et al. Preparation of epalrestat [P]. JP08291155, 1997-10-21.). At the same time, the research on thiazole compounds in medicine has also attracted more and more interest from chemists (Sebrell, L.B.; Boord, C.E. Preparation and properties of 1-mercaptobenzothiazole its ho...
Claims
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More Application Information
Patent Timeline
 Login to View More
Login to View More IPC IPC(8): C07D277/82A01N43/78A01P1/00
Inventor 宋宝安李为华杨松胡德禹金林红魏学范会涛薛伟李良
Owner GUIZHOU UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com