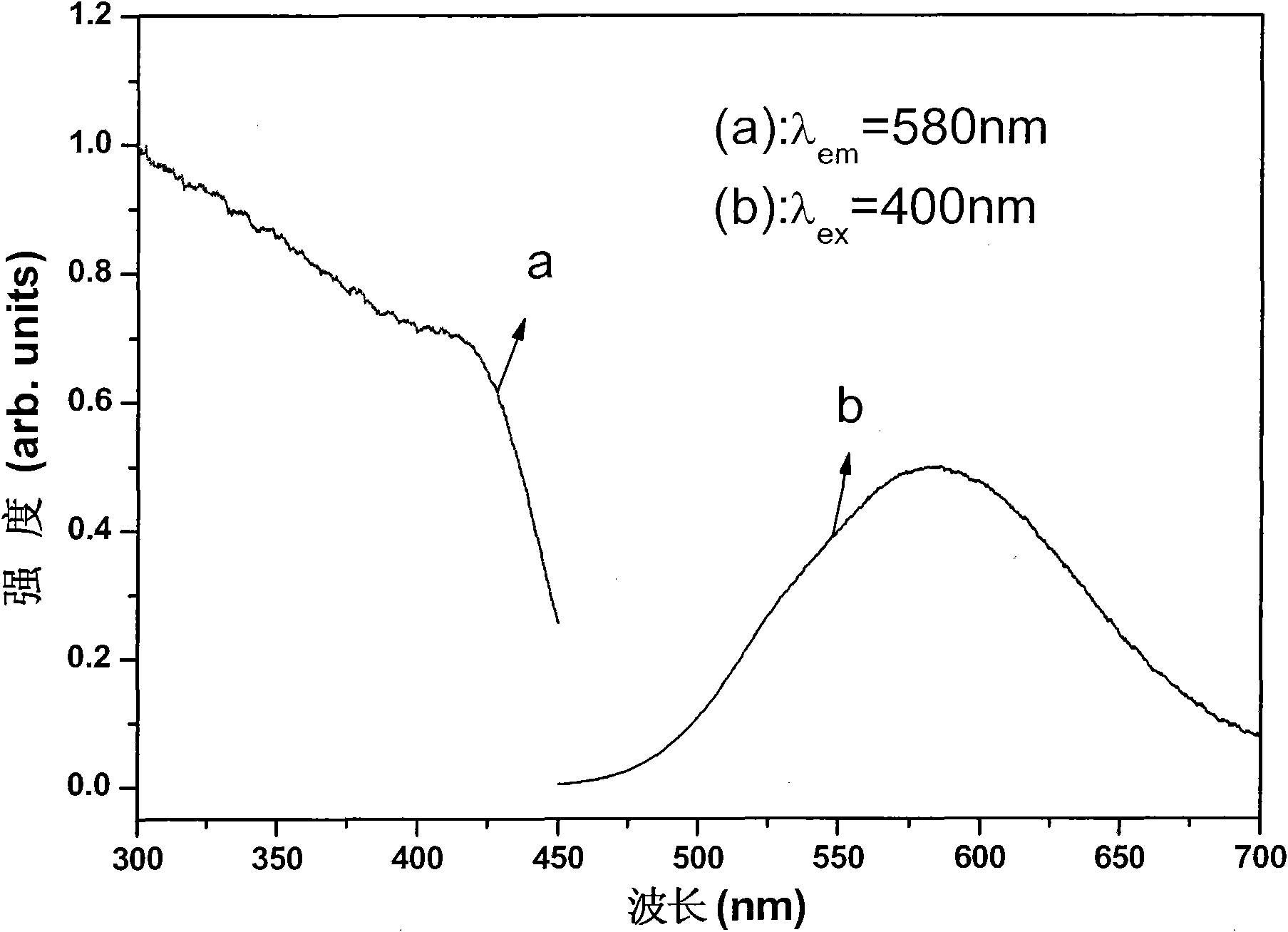
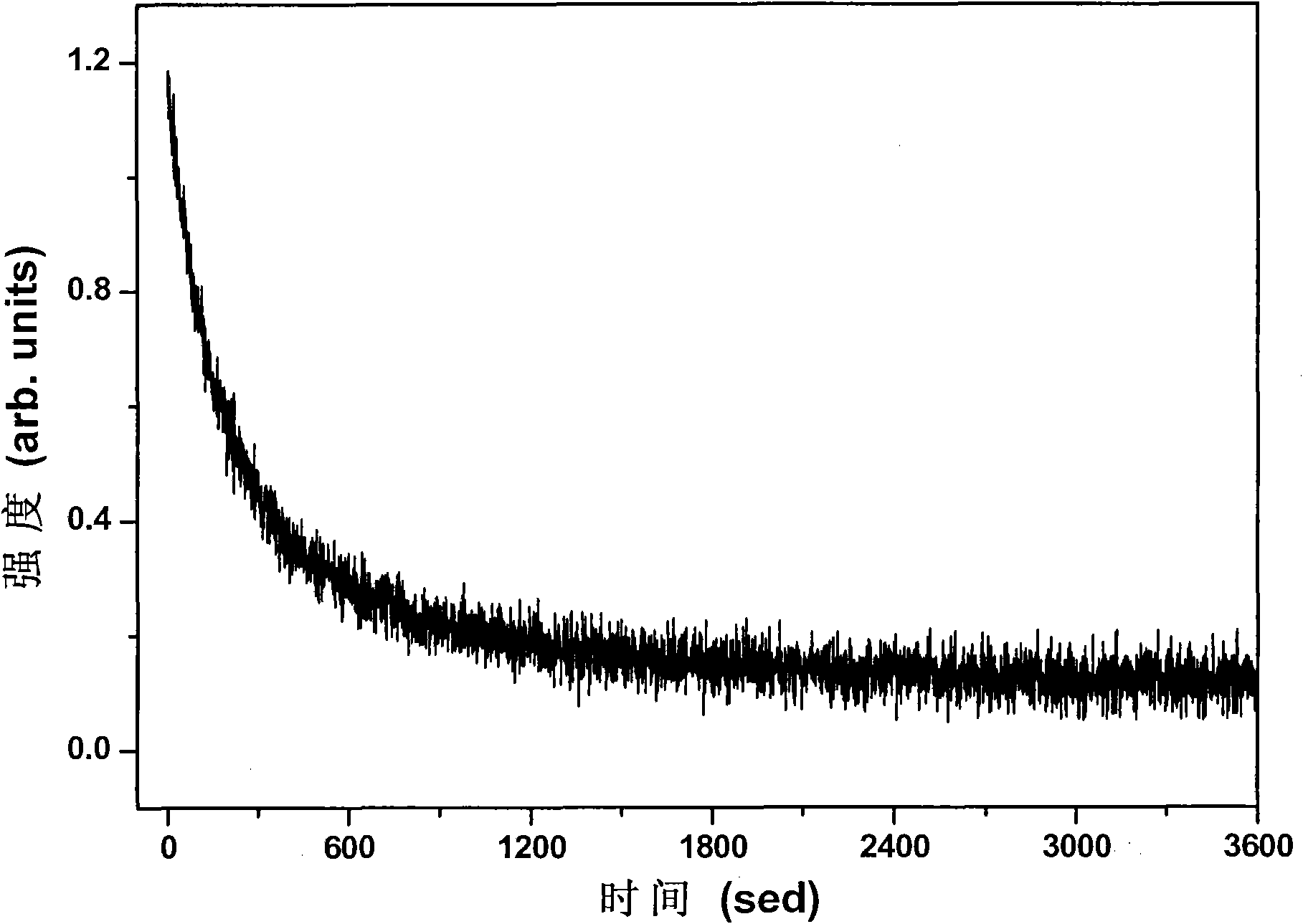
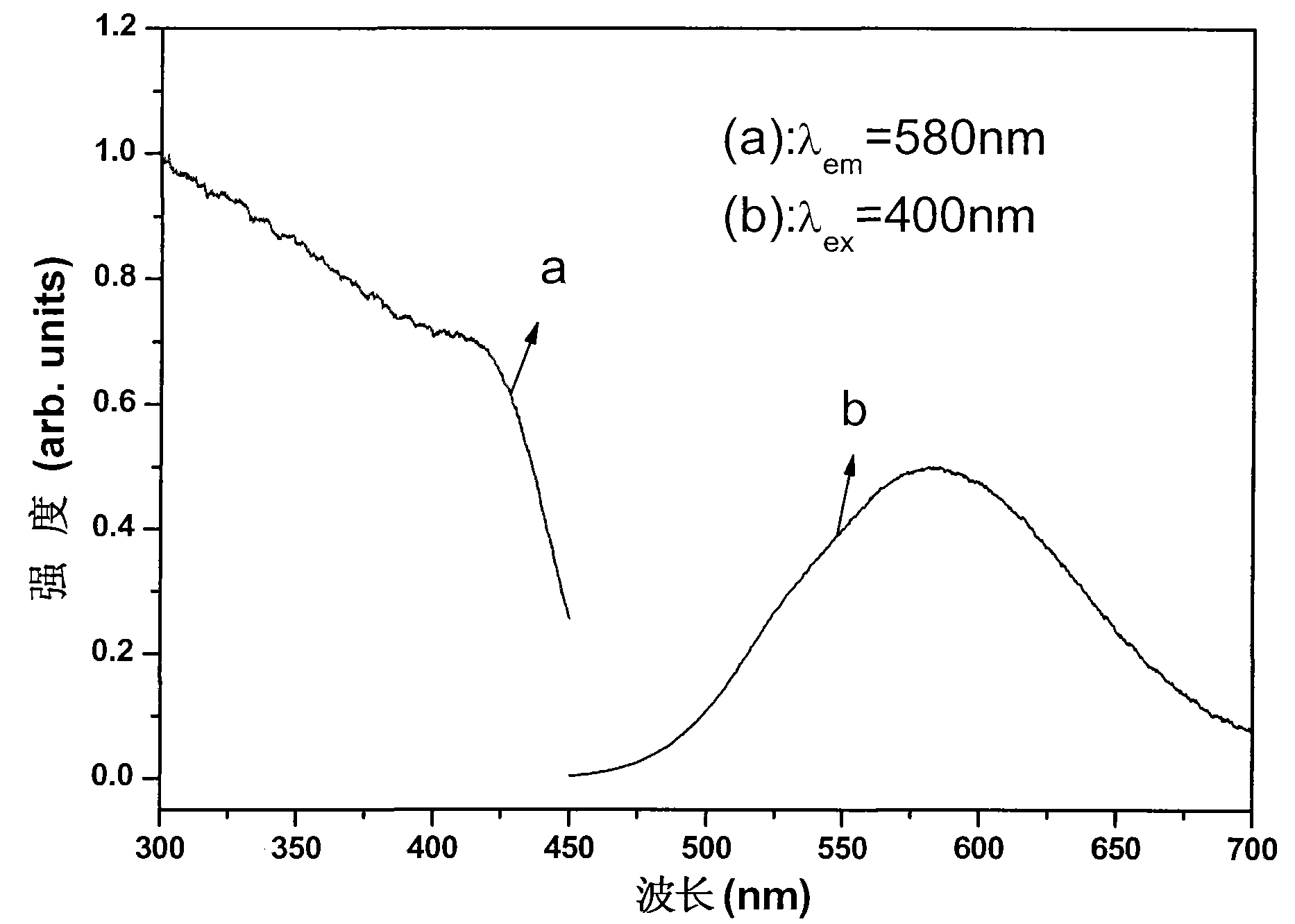
Orange long-afterglow fluorescent powder and preparation method thereof
A long-lasting phosphor and orange-yellow technology, applied in chemical instruments and methods, luminescent materials, etc., can solve the problems that the luminous brightness and afterglow time do not meet the practical requirements, the preparation process is not easy to control, pollute the environment, etc., and the preparation method is simple , Low cost of raw materials, wide excitation spectrum
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0018] Preparation of Sr 2.9985 al 2 o 5 Cl 2 :Eu 2+ 0.0005 , Dy 3+ 0.0010 (i.e. when Sr 3-x-y-z m z al 2 o 5 Cl 2 :Eu 2+ x , Dy 3+ y When z=0, x=0.0005, y=0.0010) phosphor powder.
[0019] Weigh SrCO according to stoichiometric ratio 3 1.4014 g, SrCl 2 .6H 2 O1.4664 grams (due to SrCl 2 .6H 2 O has a low melting point (873°C) and is volatile, and the calculated excess is 10%), Al 2 o 3 0.5100 g, Eu 2 o 3 0.0004 g, Dy 2 o 3 0.0009 g, mixed and ground in an agate mortar; put into a corundum crucible with a cover, placed in a high temperature furnace, under CO or H 2 Under reducing atmosphere, burn at 1200-1350°C for 3-5 hours, cool naturally and then grind to obtain orange-yellow long-lasting phosphor Sr 2.9985 al 2 o 5 Cl 2 :Eu 2+ 0.0005 , Dy 3+ 0.0010 .
Embodiment 2
[0021] Preparation of Sr 2.997 al 2 o 5 Cl 2 :Eu 2+ 0.001 , Dy 3+ 0.002 (i.e. when Sr 3-x-y-z m z al 2 o 5 Cl 2:Eu 2+ x , Dy 3+ y When z=0, x=0.001, y=0.002) phosphor powder.
[0022] Weigh SrCO according to stoichiometric ratio 3 1.4003 g, SrCl 2 .6H 2 O 1.4664 g (due to SrCl 2 .6H 2 O has a low melting point (873°C) and is volatile, and the calculated excess is 10%), Al 2 o 3 0.5100 g, Eu 2 o 3 0.0009 g, Dy 2 o 3 0.0019 g, mixed and ground in an agate mortar; put into a corundum crucible with a cover, placed in a high temperature furnace, under CO or H 2 Under reducing atmosphere, burn at 1200-1350°C for 3-5 hours, cool naturally and then grind to obtain orange-yellow long-lasting phosphor Sr 2.997 al 2 o 5 Cl 2 :Eu 2+ 0.001 , Dy 3+ 0.002 .
Embodiment 3
[0024] Preparation of Sr 2.97 al 2 o 5 Cl 2 :Eu 2+ 0.01 , Dy 3+ 0.02 (i.e. when Sr 3-x-y-z m z al 2 o 5 Cl 2 :Eu 2+ x , Dy 3+ y When z=0, x=0.01, y=0.02) phosphor powder.
[0025] Weigh SrCO according to stoichiometric ratio 3 1.3803 g, SrCl 2 .6H 2 O1.4664 grams (due to SrCl 2 .6H 2 O has a low melting point (873°C) and is volatile, and the calculated excess is 10%), Al 2 o 3 0.5100 g, Eu 2 o 3 0.0088 g, Dy 2 o 3 0.0187 g, mixed and ground in an agate mortar; put it into a corundum crucible, cover it, place it in a high-temperature furnace, and burn it at 1200-1350°C for 3-5 hours in a reducing atmosphere of CO or H2; after natural cooling Grind to get orange long afterglow phosphor Sr 2.97 al 2 o 5 Cl 2 :Eu 2+ 0.01 , Dy 3+ 0.02 .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com