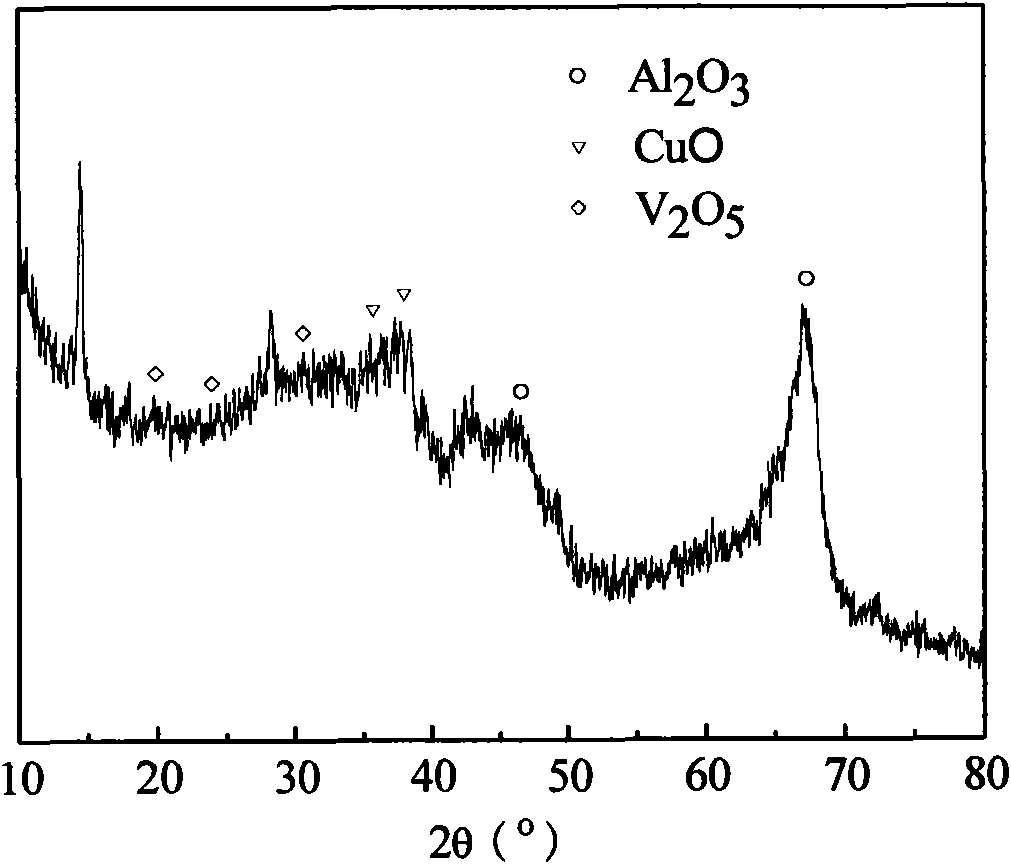
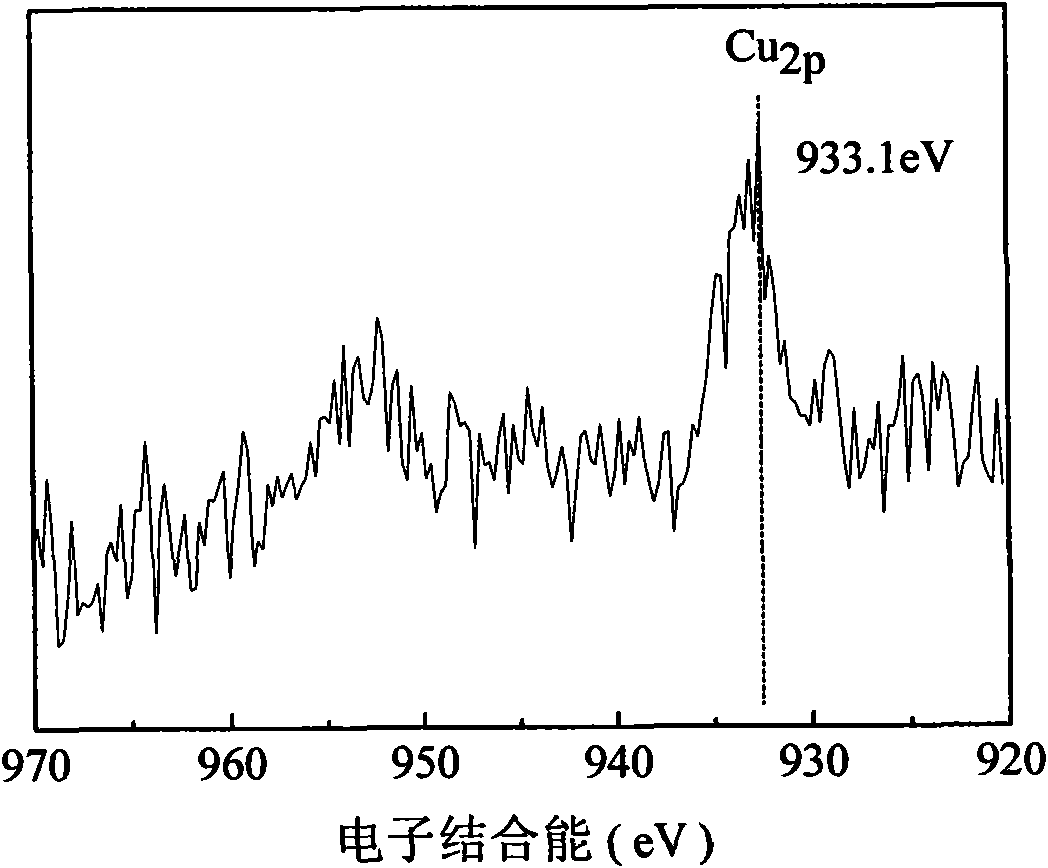
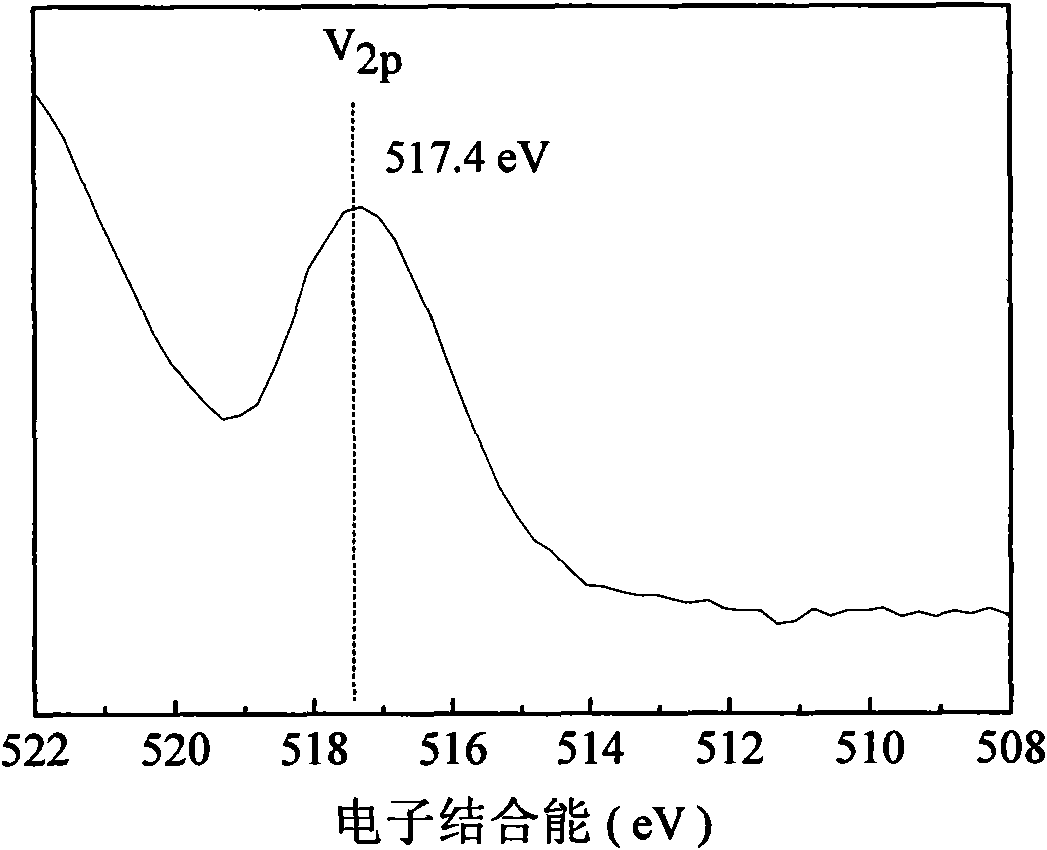
Catalyst for synthesizing methylaniline from methylbenzene by one step and preparation method thereof
A technology of methylaniline and catalyst, applied in the field of aromatic hydrocarbon amination reaction synthesis, can solve the problems of large consumption, difficult product separation, unrecyclable homogeneous catalyst, etc., and achieves less usage, easily available raw materials, and a preparation process. simple effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] (1) Select the active components CuO and V in the supported catalyst for the synthesis of methylaniline 2 O 5 The load capacity is 16.4%;
[0040] (2) Weigh 5g 20~40 mesh γ-Al 2 O 3 , Put it in a 100ml evaporating dish, use deionized water to measure the volume required for equal volume immersion to 5ml;
[0041] (3) CuO and V as the active components selected in step (1) 2 O 5 The loading amount is 16.4% to determine the required CuO and V 2 O 5 The total amount is 0.98g; according to CuO and V 2 O 5 The molar ratio of 0.25:1 to calculate the required precursor Cu(NO 3 ) 2 1.21mmol and NH 3 VO 3 9.70mmol;
[0042] (4) Weigh 5ml of deionized water determined in step (2), add 14.56mmol(COOH) 2 ·2H 2 O powder, mixed into a colorless and transparent oxalic acid solution;
[0043] (5) The NH determined in step (3) 3 VO 3 Add to the oxalic acid solution obtained in step (4) above to form a blue-green immersion solution;
[0044] (6) Weigh the catalyst carrier with the same mass as in...
Embodiment 2~4
[0056] The other steps are the same as in Example 1, except that the carrier used in the catalyst is silica, HZSM-5 molecular sieve (SiO 2 / Al 2 O 3 =135), titanium oxide, to prepare supported CuO-V for synthesis of methylaniline 2 O 5 Catalyst, in which active components CuO and V 2 O 5 The loading capacity is 16.4%.
[0057] Catalyst activity evaluation: Weigh 0.2 g of the supported catalysts prepared in Examples 2 to 4 into three three-necked flasks for performance evaluation. The reaction conditions are the same as the catalyst evaluation conditions in Example 1. The results of the reaction are shown in Table 2.
[0058] Table 2 The influence of carrier on catalyst performance
[0059] Example
Embodiment 5
[0061] (1) Select the active components CuO and V in the supported catalyst for the synthesis of methylaniline 2 O 5 The load capacity is 5.5%;
[0062] (2) Weigh 5g 20~40 mesh γ-Al 2 O 3 , Put it in a 100ml evaporating dish, use deionized water to measure the volume required for equal volume immersion to 5ml;
[0063] (3) CuO and V as the active components selected in step (1) 2 O 5 The loading amount is 5.5% to determine the required CuO and V 2 O 5 The total amount is 0.29g; according to CuO and V 2 O 5 The molar ratio of 0.25:1 to calculate the required precursor Cu(NO 3 ) 2 0.36mmol and NH 3 VO 3 2.90mmol;
[0064] (4) Weigh 5ml of deionized water determined in step (2), add 4.36mmol(COOH) 2 ·2H 2 O powder, mixed into a colorless and transparent oxalic acid solution;
[0065] Steps (5) to (10) are the same as in Example 1 to prepare supported CuO-V for the synthesis of methylaniline 2 O 5 Catalyst, in which active components CuO and V 2 O 5 The loading capacity is 5.5%.
[0066] C...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com