Cyclic hexapeptide antibacterial compound
A cyclohexapeptide and antibacterial drug technology, applied in the field of novel cyclohexapeptide antibacterial compounds, can solve problems such as differences in amino acid composition, and achieve good inhibitory activity.
Active Publication Date: 2010-09-29
海南博士威生物科技有限公司
View PDF0 Cites 10 Cited by
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
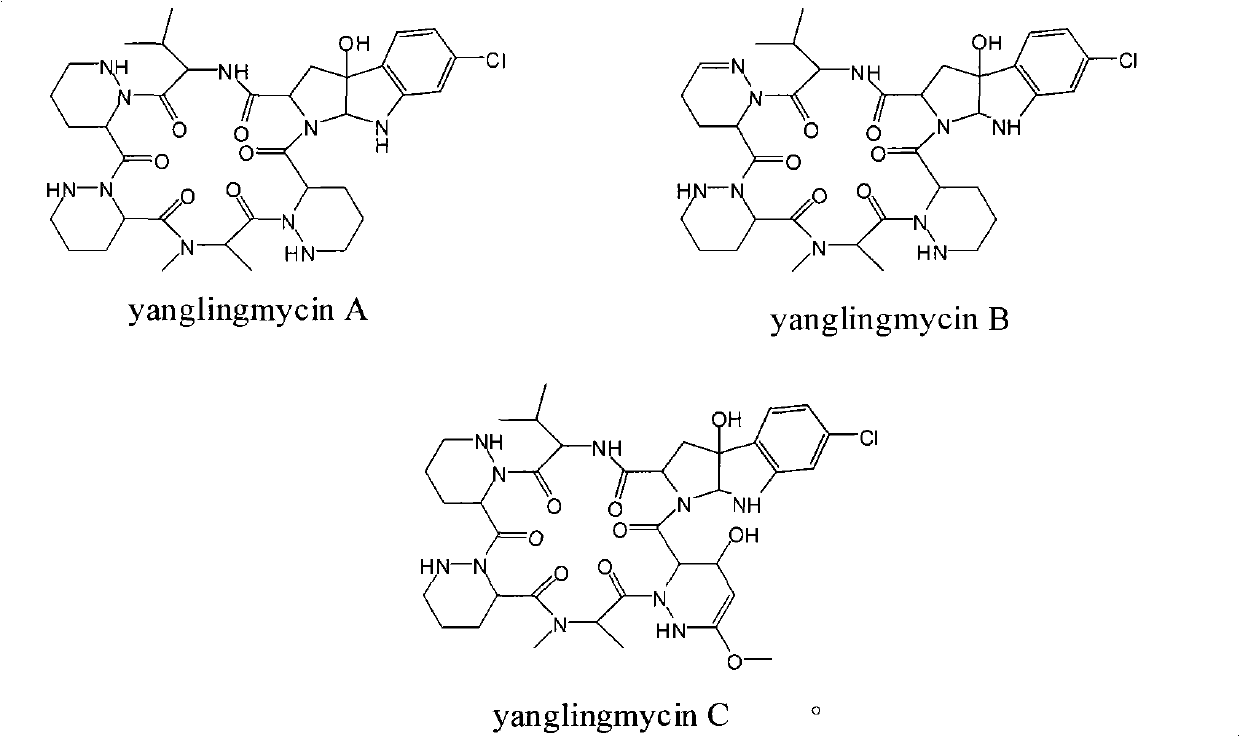
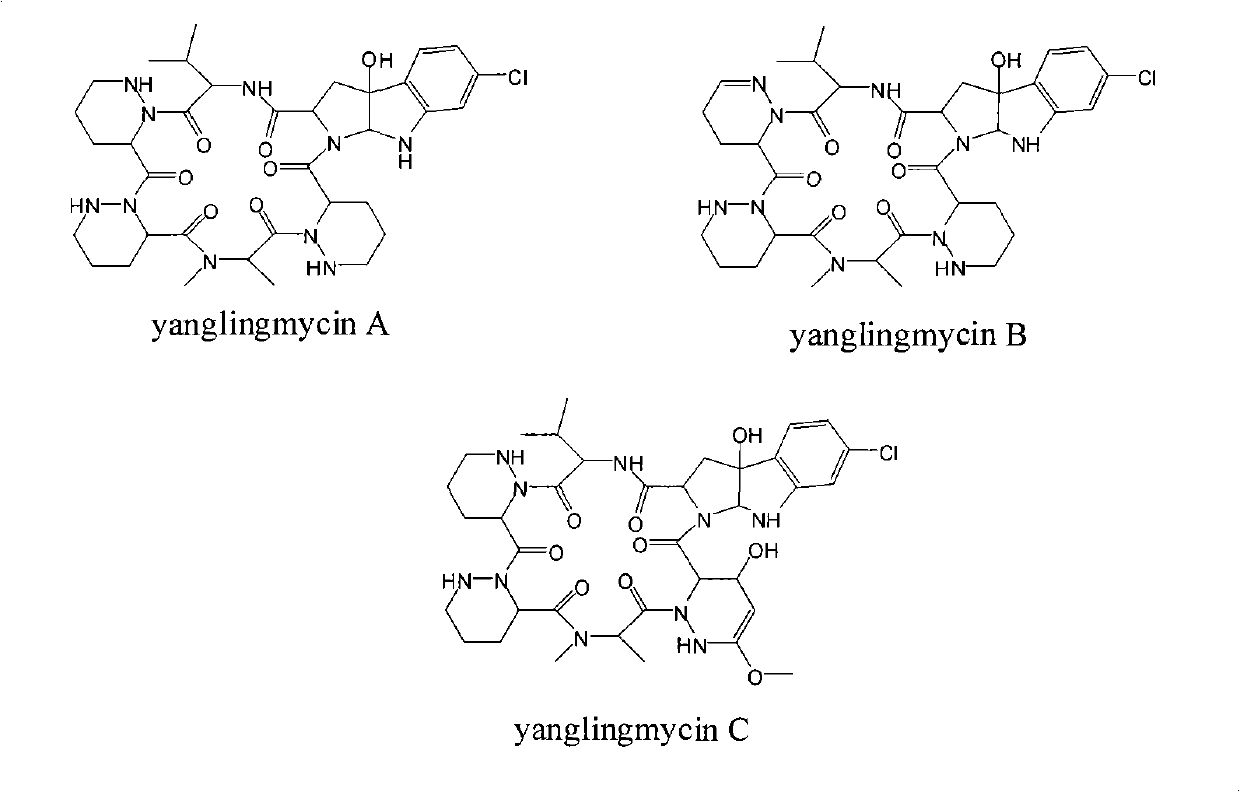
The cyclic hexapeptide antibiotics similar to this structure have been found so far, including himastatin and chloptosin, but the latter two are dimers formed by two cyclic hexapeptides, and there are also differences in amino acid composition
Method used
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
View moreImage
Smart Image Click on the blue labels to locate them in the text.
Smart ImageViewing Examples
Examples
Experimental program
Comparison scheme
Effect test
Embodiment 1
Embodiment 2
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More PUM
 Login to View More
Login to View More Abstract
The invention provides a novel cyclic hexapeptide antibacterial compound yanglingmycins, belonging to the technical field of medicaments. The compound in the invention has a structural formula shown in the specification. The amino acid composition of the cyclic hexapeptide antibacterial compound comprises valine and other unconventional amino acids, such as 2-methylamino propionic acid, piperazin-3-carboxylic acid and 6-chloro-3alpha-hydroxy-1, 2, 3, 3alpha, 8, 8alpha-hexyhydro [2, 3-pyrrolo] indol-2-carboxylic acid, wherein each amino acid is in an L configuration or a D configuration. The compound in the invention can strongly inhibit many kinds of gram positive bacteria such as bacillus cereus, bacillus subtilis, staphylococcus aureus and methicillin resistant staphylococcus aureus.
Description
Technical field: The invention relates to the technical field of medicines, in particular to a novel cyclic hexapeptide antibacterial compound yanglingmycins extracted and separated from soil actinomycete fermentation broth. Background technique: Screening biologically active substances from microbial secondary metabolites is an important approach in the research and development of new drugs. So far, there are more than 50,000 microbial secondary metabolites whose structures have been clarified, including more than 22,000 compounds with clear biological activities. Since Wakesman isolated streptomycin from the culture solution of Streptomyces griseus, soil actinomycetes have become the main source of antibiotics, and the amount of antibiotics produced by them accounts for 47% of all microbial active metabolites. Yanglingmycins is a new class of cyclic hexapeptide antibacterial compounds produced by the metabolism of Streptomyces alboflavus No.313 isolated from the soil of ...
Claims
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More Application Information
Patent Timeline
 Login to View More
Login to View More IPC IPC(8): C07K7/64A61K38/12A61P31/04
Inventor 吴文君姬志勤郭正彦
Owner 海南博士威生物科技有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com