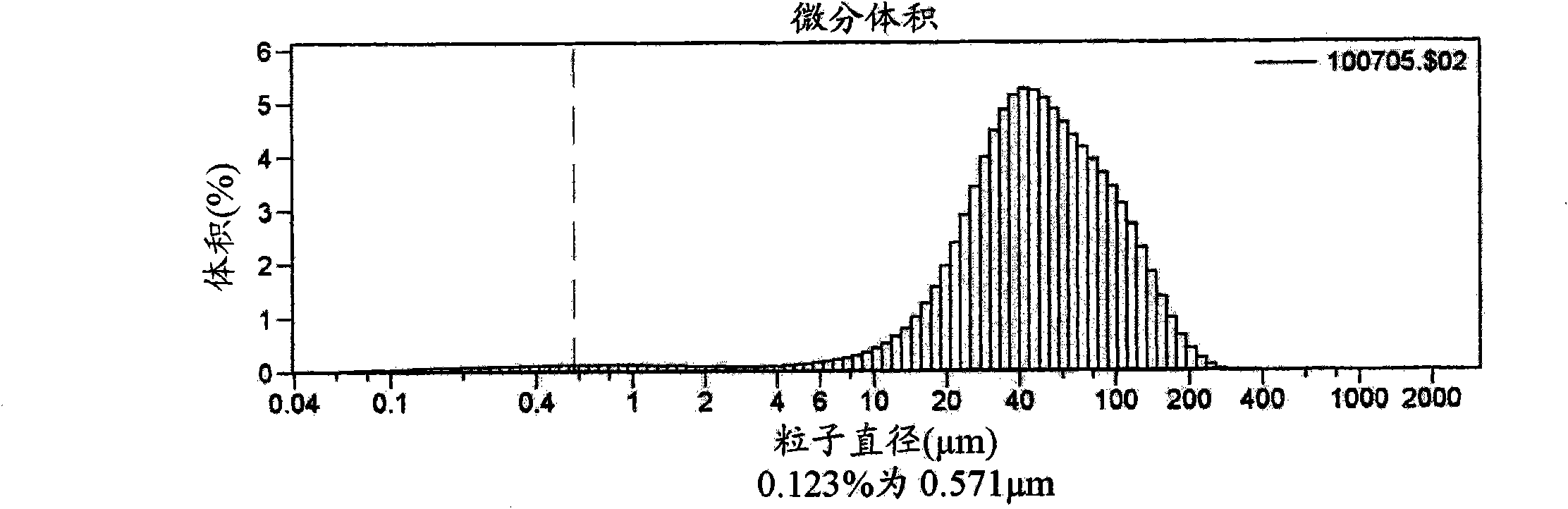
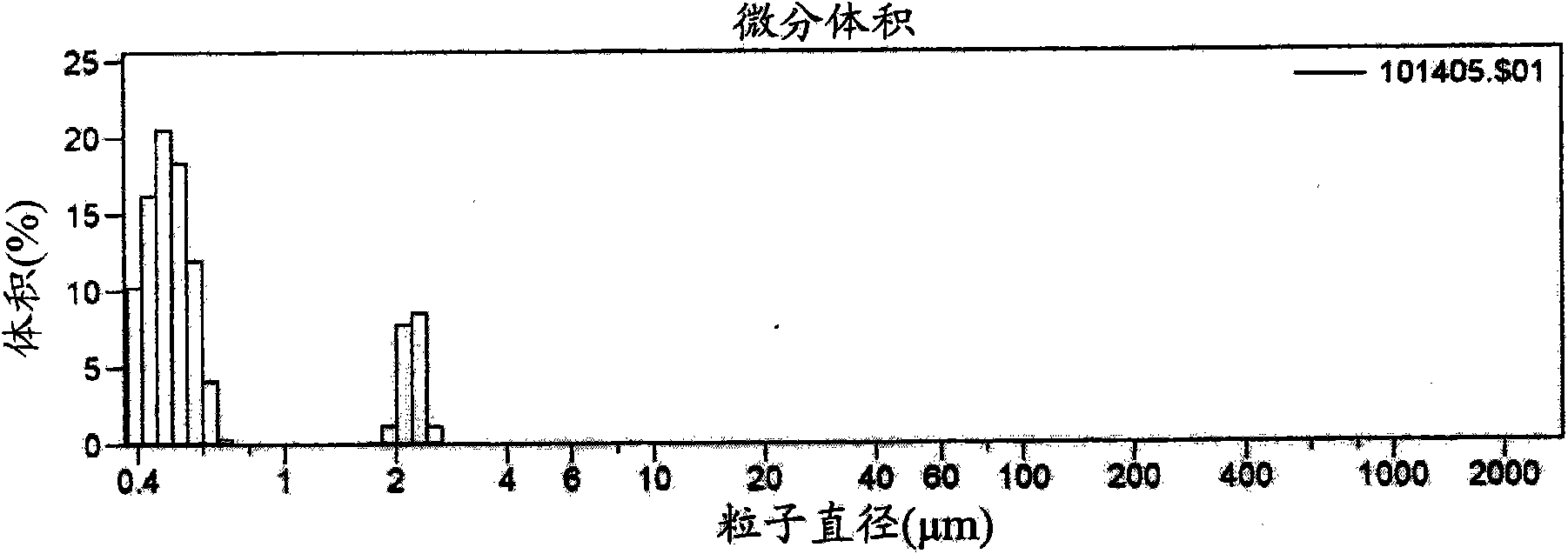
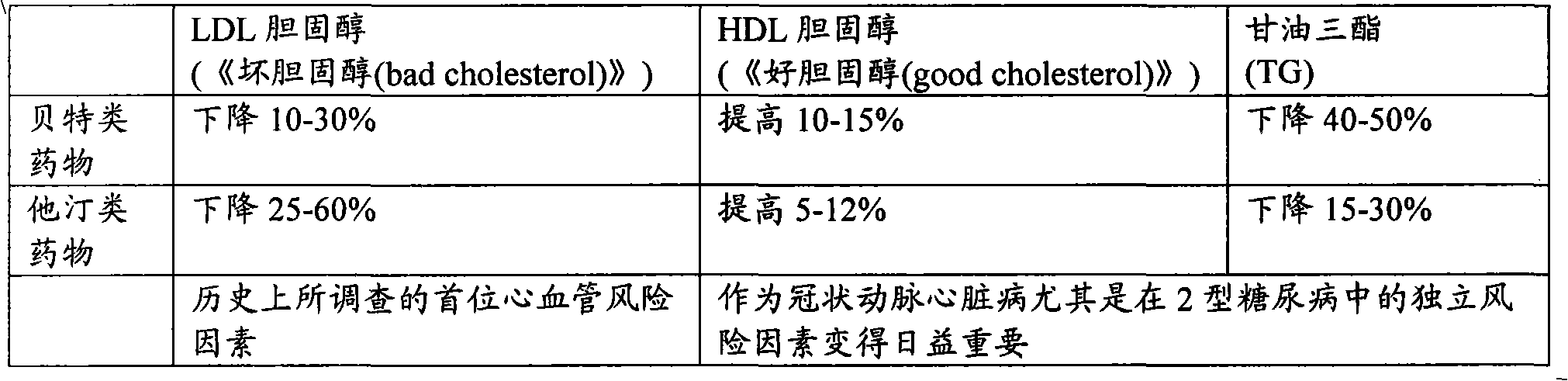
Multi-phasic, nano-structured compositions containing a combination of a fibrate and a statin
A composition, fibrate technology, applied in the direction of drug combination, drug delivery, medical preparations containing active ingredients, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0100] Preparation I: Control Substance
[0101] Non-micronized fenofibrate powder was suspended in hydroxypropylmethylcellulose (HPMC, grade E4M) to obtain a suspension with a concentration of 0.5 wt% (48 mg fenofibrate / g suspension). The suspension is mixed very well to obtain a homogeneous suspension free of lumps and / or aggregates.
[0102] Formulation II: Standard
[0103] TriCor tablets (48 mg fenofibrate / tablet) (from Abbott Pharma) were ground using a mortar and pestle until a mass free of aggregates was obtained. Then, the material was suspended in 1 ml of purified water to obtain a homogeneous suspension.
[0104] Formulation III: Test Article I
[0105] Fenofibrate (4.8 g) was mixed with ethanol (8.8 g), polysorbate 80 (9.4 g) and soybean oil (50.2 g). Water (26.8 grams) was added and the whole mixture was emulsified using a paddle stirrer. The resulting emulsion was then subjected to three rounds of high-pressure homogenization (APV-1000) at 10,000 psi (...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com