Application of human urinary kallidinogenase in preparing medicine for treating diabetic nephropathy
A technique for urinary kininogenase and diabetic nephropathy, which is applied in the field of preparation of drugs for treating diabetic nephropathy, and can solve problems such as the reduction of kininogenase activity and the effect on vascular regulation function
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0012] Example 1 Preparation of human urinary kininogenase dry powder injection
[0013] Take filter-sterilized human urinary kininogenase 150PNA units, add 7.5 grams of mannitol, 2 grams of dextran 40, and 5 grams of sodium citrate to dissolve, adjust the pH to neutral, add water for injection to 500 ml, sterile filter, divide Put it in 1000 ampoules, and freeze-dry it under aseptic conditions.
Embodiment 2
[0014] Example 2 Preparation of human urinary kininogenase injection
[0015] Take filtered sterilized human urinary kininogenase dissolved in 150 PNA units, adjust the pH to neutral, add water for injection to 500 ml, add sodium chloride to adjust isotonicity, filter aseptically, divide into 1000 ampoules, and obtain.
Embodiment 3
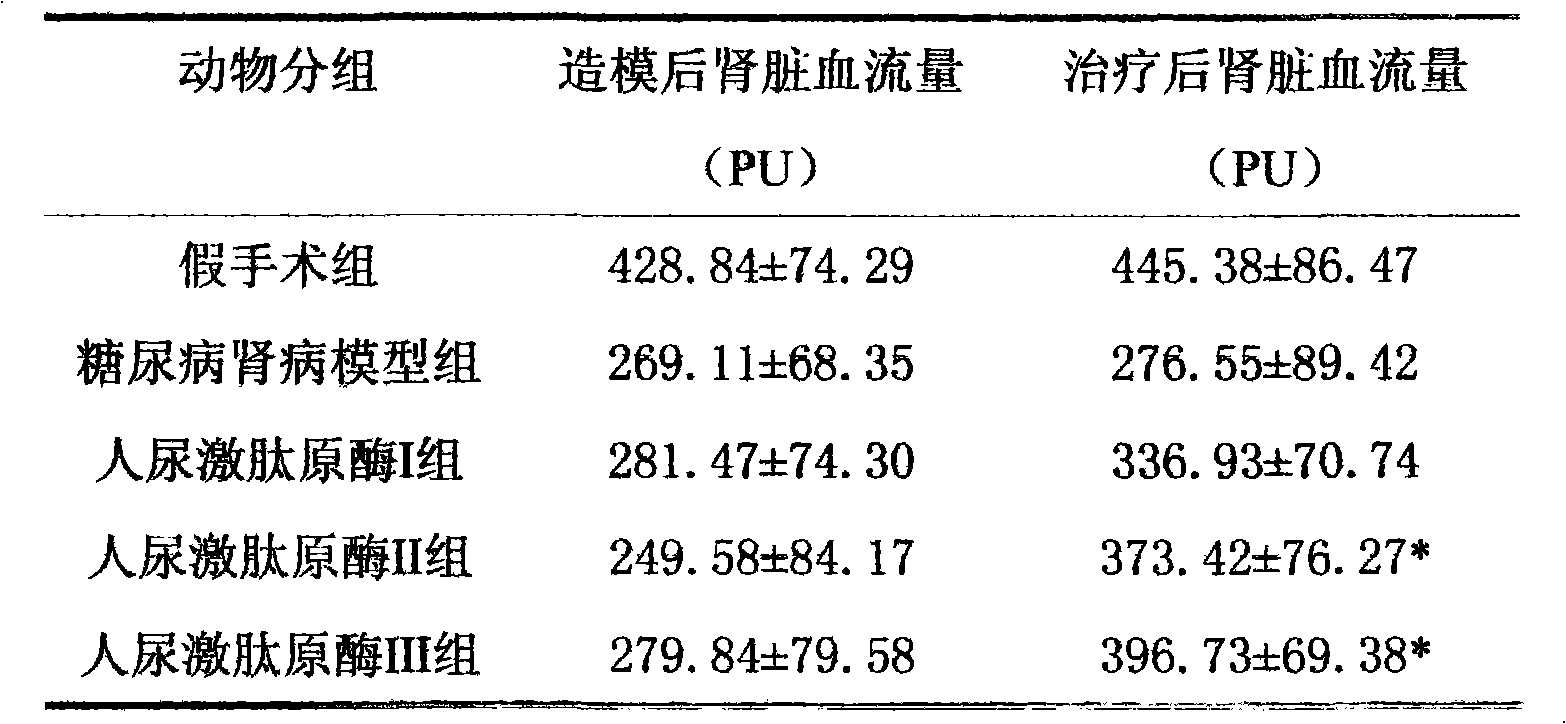
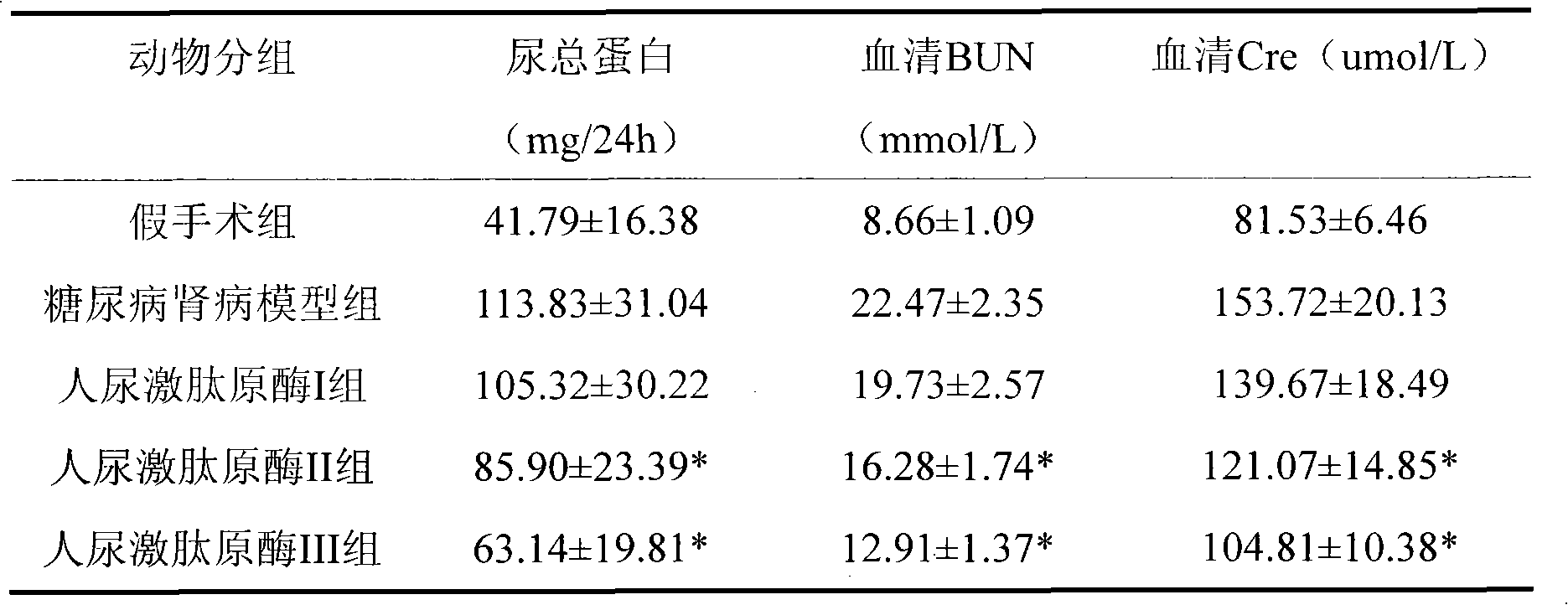
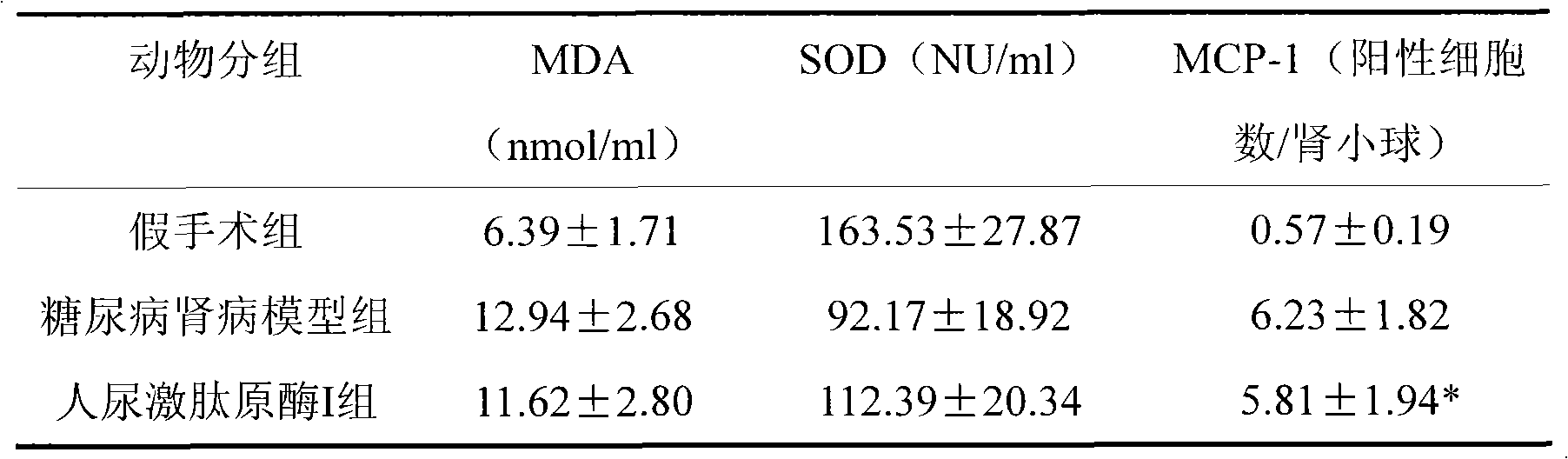
[0016] Example 3 Therapeutic effect of human urinary kallikrein on rats with diabetic nephropathy
[0017] experimental method
[0018] Rat animal model of diabetic nephropathy induced by unilateral nephrectomy plus intraperitoneal injection of streptozotocin (STZ). Select 60 SD rats of 180-220g, male or female, and randomly divide them into 5 groups: sham operation group; DN model group; human urinary kallikrein I group (3.85×10 -3 PNAu); human urinary kininogenase II group (8.75×10 -3 PNAu); human urinary kininogenase III group (17.5×10 -3 PNAu), rats in each treatment group were injected with human urokininogenase into the tail vein after modeling, once a day for 4 consecutive weeks. The sham operation group and the DN model group were given the same volume of saline through the tail vein.
[0019] Observation indicators
[0020] After 4 weeks, the 24-h urine of the rats in each group was collected and stored at -20°C for 24-h urine protein testing. After urination, t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com