Purification method of pyrrolidine carbapenem antibiotics
A purification method and technology of aqueous solution, applied in the fields of organic chemistry, anti-infective drugs, pharmaceutical formulations, etc., can solve the problems of residual by-products, darkening of color, low product purity, etc., and achieve the effect of increasing stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
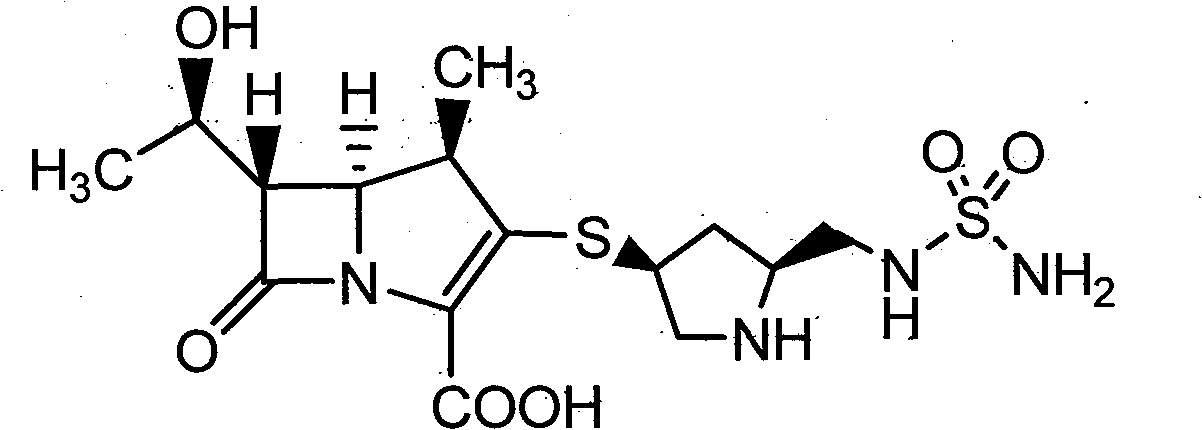
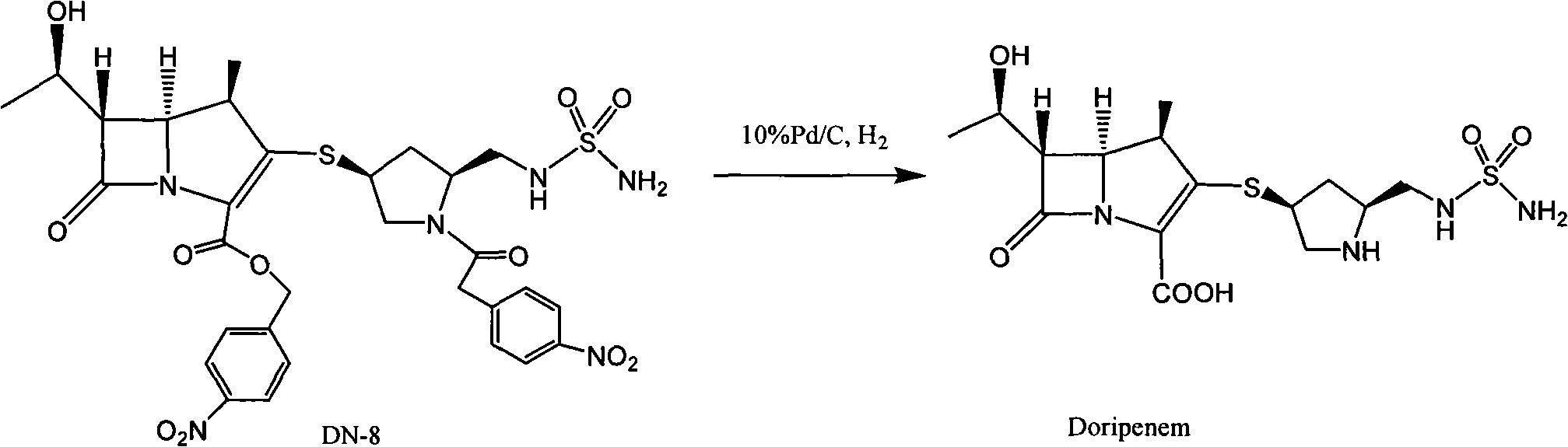
[0036] Embodiment 1: Preparation of crude product of doripenem
[0037]
[0038] 100 grams of DN-8 (refer to literature organic process research & development 2003, 7, 649-654 method preparation) was dissolved in water (450mL) and THF (800mL), added 100 grams of Pd / C (water content 66%), heated to At 25°C, under the pressure of 20-24kg, react for 2 hours, exotherm during hydrogenation, keep the reaction temperature at 30-40°C, after the reaction is completed, filter with suction, and wash the palladium with 150mL (THF:water=2:1) Carbon, wash the water layer with ethyl acetate (750mL×2), wash the water layer with n-butanol (500mL×2), cool the water layer to 0°C, a light yellow solid precipitates, keep warm at 0-5°C and stir overnight, then filter with suction , dried under reduced pressure at 25° C. for 15 hours, crude doripenem (32 g, yield 65%, water content 6.1%, purity 96.2%).
Embodiment 2
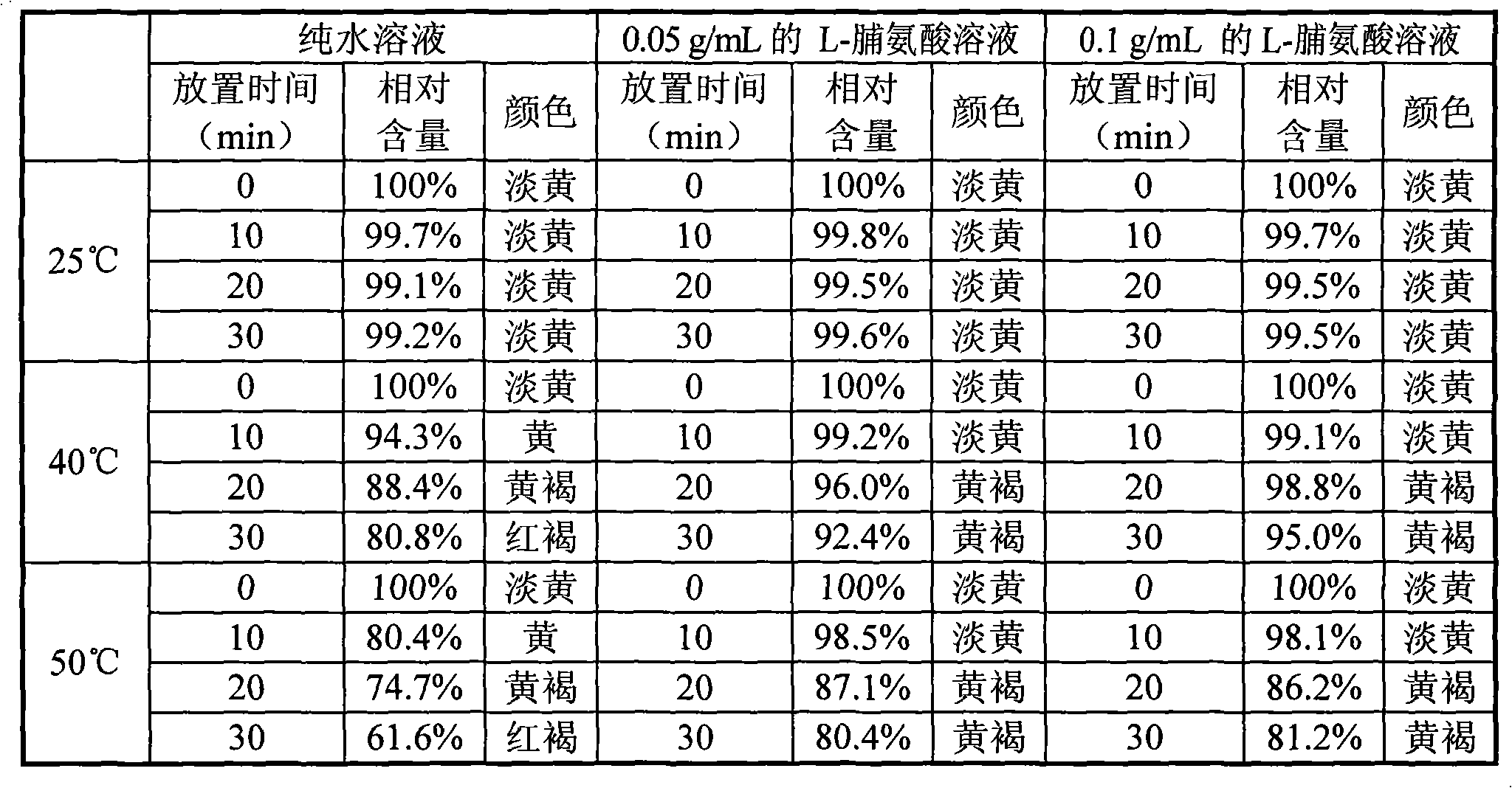
[0039] Embodiment 2: solution stability investigation
[0040]Take 1 gram of crude doripenem, dissolve it in 50ml of pure water, 0.05g / mL L-proline aqueous solution, and 0.1g / mL L-proline aqueous solution, stir and dissolve until a clear transparent liquid is obtained. Take 10ml and place them at room temperature at 25°C, 40°C, and 50°C, respectively take 10min, 20min, and 30min of the solution for HPLC detection, use the peak area at room temperature at 0min as a control, calculate the relative peak area ratio, and calculate the relative content. To examine its solution stability. It can be seen from the table below that the stability of doripenem in solution is significantly improved at higher temperatures (40° C. and 50° C.) after adding proline.
[0041]
Embodiment 3
[0043] Add the crude product of doripenem (1.0g) into 0.05g / mL L-proline solution (20mL), heat with stirring, and dissolve at 50°C, keep the solution at 50-55°C, add activated carbon ( 50 mg) was stirred for 5 minutes to decolorize, filtered, and the filtrate was cooled to 0-5°C and stirred. Solids precipitated within about 1 hour, and aged at 0-5°C for 2 hours. 10 mL of isopropanol was added dropwise, aged at 0-5°C for 2 hours, stirred overnight at -10°C, and the resulting crystals were filtered out. The obtained crystals were washed with 80% isopropanol aqueous solution (5 mL), and dried under reduced pressure at 30° C. with a water pump (20-30 mmHg) for 4 hours to obtain 0.91 g of doripenem (91% yield, 5.93% water content) , Purity: 99.65%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com