Synthesis method of m-phenoxytoluene
A m-phenoxytoluene, synthetic method technology, applied in the formation/introduction of ether group/acetal group/ketal group, ether preparation, organic chemistry, etc., can solve the problems of long steps and low total yield, etc. To achieve the effect of cost reduction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
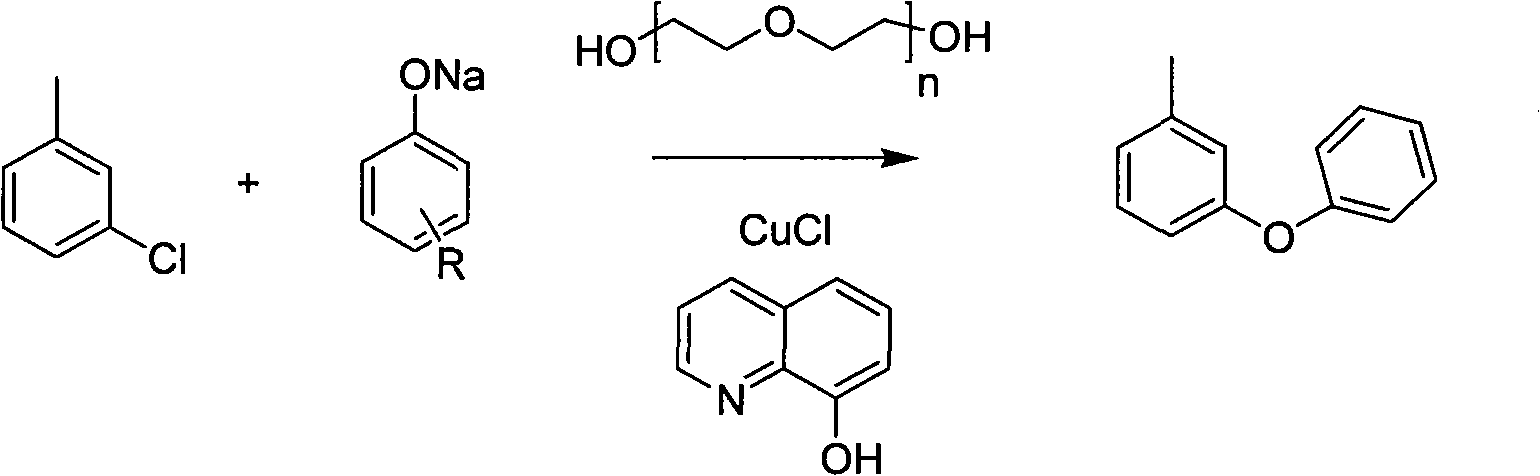
[0012] Concrete reaction formula of the present invention is:
[0013]
[0014] In the formula, It means polydiethylene glycol, and any degree of polymerization can play a catalytic effect. The reaction condition is heating. The reaction equation shows: different substituted sodium phenates suitable for this method.
[0015] Specific steps are as follows:
[0016] 1. Put 126.5g of m-chlorotoluene, 23.2g of sodium phenoxide, 42g of cuprous chloride, 4.1g of 8-hydroxyquinoline, and 4.0g of polydiethylene glycol into a 500mL round bottom flask, and stir at room temperature for 5±1 minutes;
[0017] 2. Heat the mixture to 130-140°C while stirring;
[0018] 3. Insulate and stir the mixture at 130-140°C for 8-9 hours;
[0019] 4. After the reaction is over, high vacuum distillation (14-16 mmHg) is carried out, and the fraction at 100-110° C. can be obtained to obtain the final compound.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com