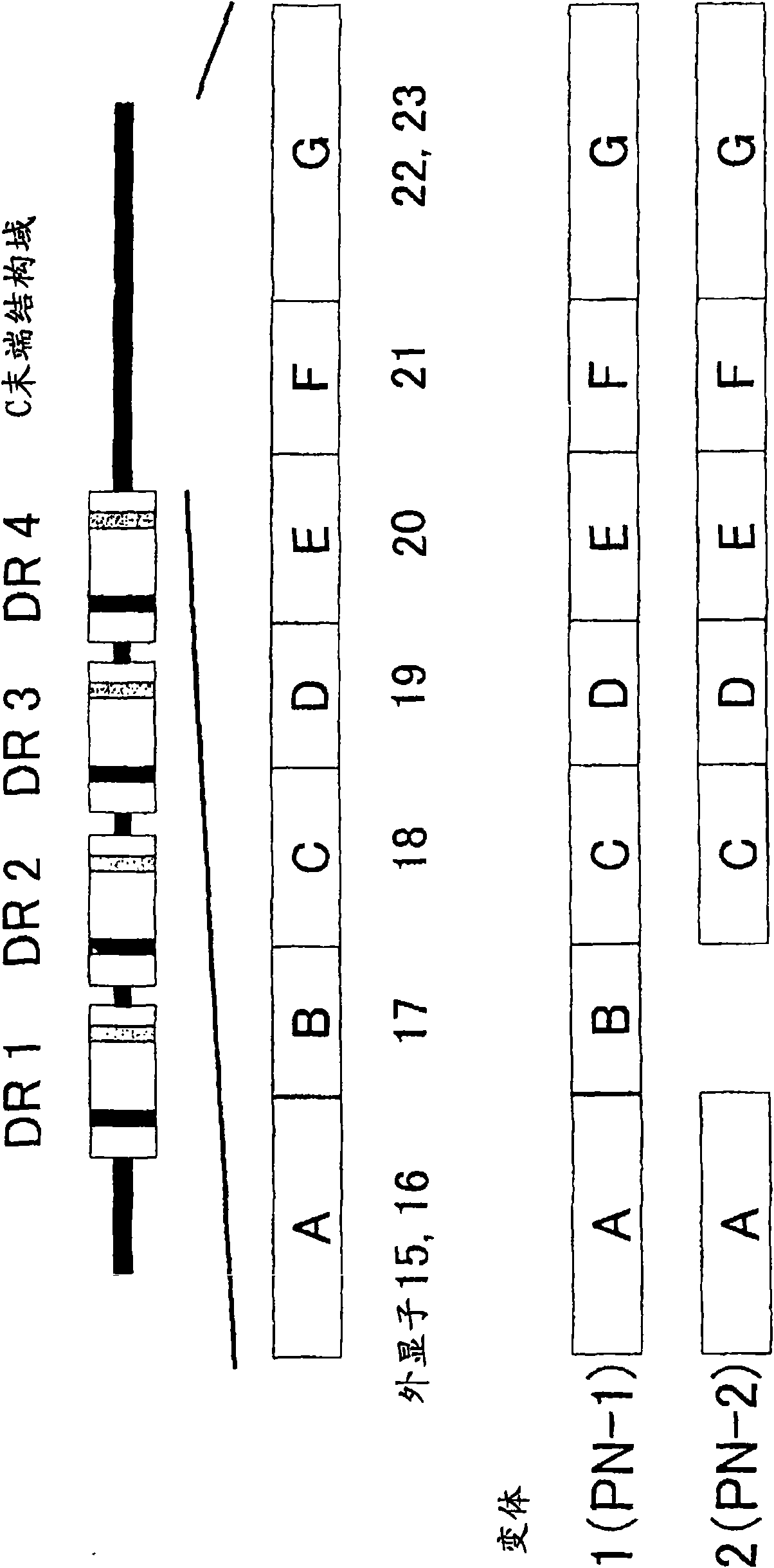
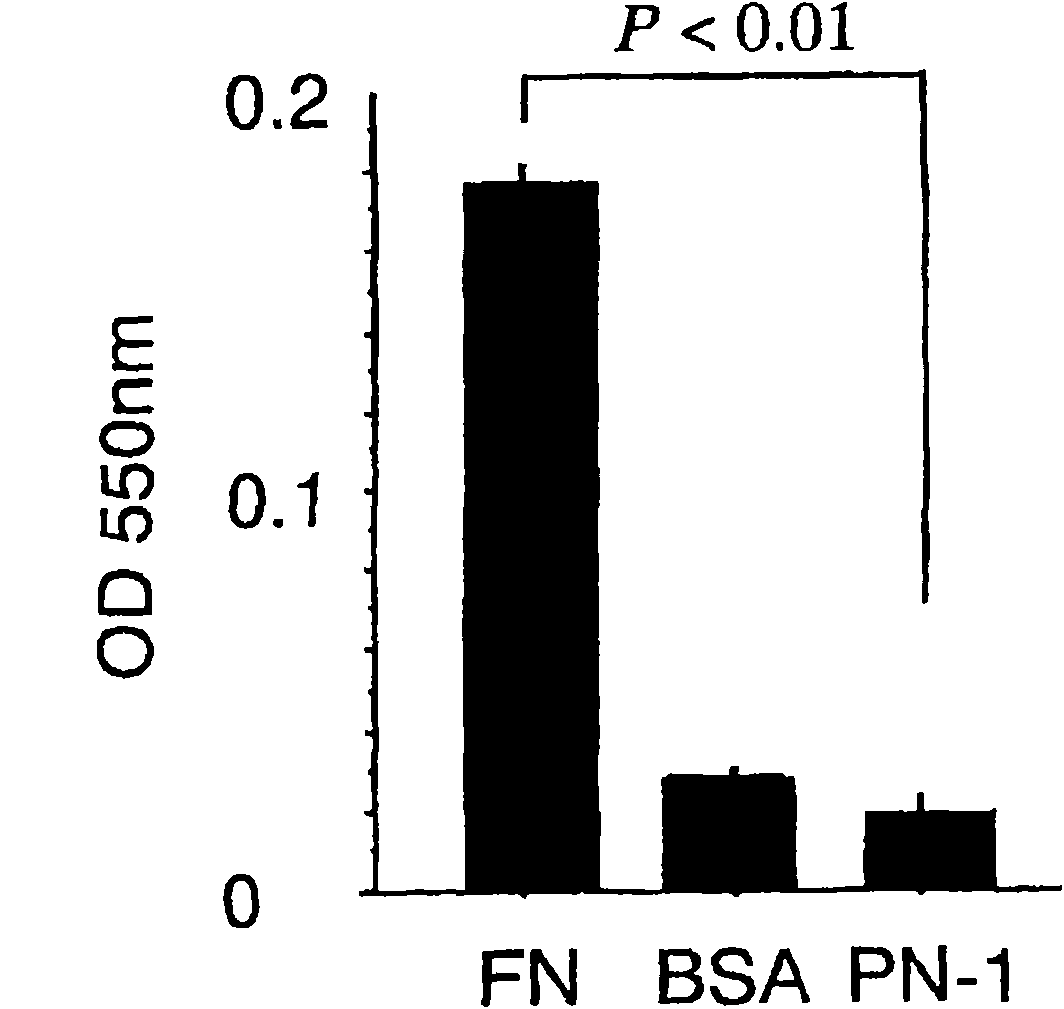
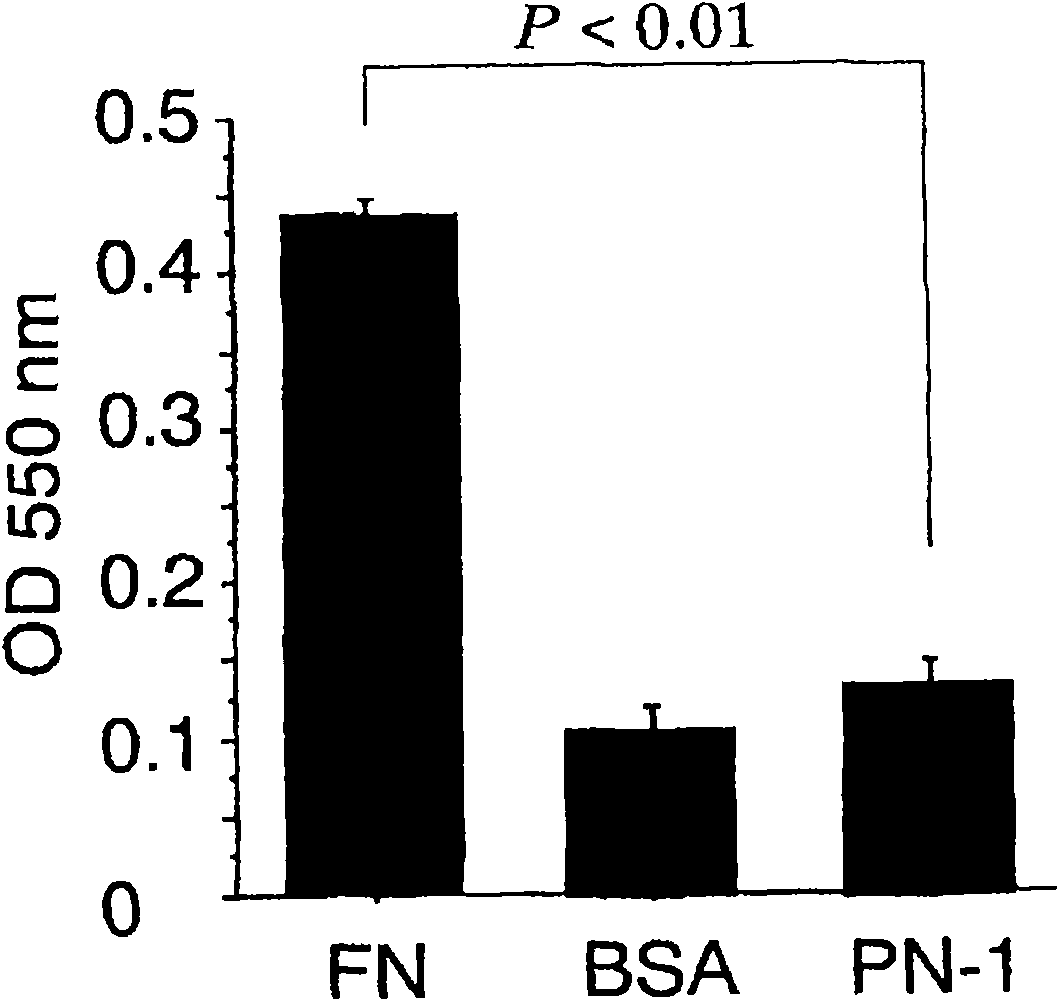
Cancer remedy containing antibody against peptide encoded by exon-17 of periostin
A cancer treatment and antibody technology, which can be used in antibody medical components, medical preparations containing active ingredients, antibodies, etc., and can solve problems such as unclear functions of splice variants.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
manufacture example 1
[0052] Hereinafter, the present invention will be described in detail and concretely using examples. Example > [manufacturing example 1] Retrieval of periostin by subtraction method 1-1 Preparation of heart failure model rats and extraction of left ventricle samples Raised with high-salt food containing 8% salt from the age of 6 weeks Male Dahl has a high salt sensitivity Rats (Dahl-S) (clear water experimental material), three left ventricle were removed in the period of cardiac hypertrophy (11 weeks) and heart failure period (14 weeks).
[0053] 1-2 Preparation of mRNA Total RNA was prepared from about 500 mg of the above-mentioned left ventricle using ISOGEN (Nippon Gene) according to the method described in the manual. Next, using the Fast Track 2.0 Kit (Invitrogen), mRNA was purified from about 400 μg of total RNA mixed with 3 animals in the cardiac hypertrophy phase and heart failure phase according to the method described in the manual, and about 3 μg of mRNA were re...
manufacture example 2
[0060] [Production Example 2] Cloning of rat periostin cDNA Isolation of rat periostin cDNA, using primers designed based on the base sequence of SF014 (1) 5'-GTTCATTGAAGGTGGCGATGGTC-3' (SEQ ID NO: 15), (2) 5'-GAGATAAAATCCCTGCATGGTCCT-3' (SEQ ID NO : 16), 10 phage subpools (subpool) (about 40,000 clones) of about 4000 clones prepared by the rat aorta cDNA library (Clontech company) inserted in the λgtII vector were carried out PCR screening, Three positive subpools were obtained. For the fragments obtained by amplifying one of the subpools by the above PCR, use AlkPhos Direct TM (Amersham Pharmacia Company), using alkaline phosphatase-labeled probes for screening by hybridization, and obtained a positive clone rat periostin #1. This insert fragment was integrated into the EcoRI site of pBluescriptII (Stragene), and the full base sequence was determined according to the method of Production Example 1-5.
[0061] The obtained clone was about 3 kb in length, corresponding to ...
manufacture example 3
[0065] [Manufacturing Example 3] Construction of Myc-His-rat periostin fusion protein expression vector An expression vector having a Myc epitope and 6 histidine tags at the carboxyl terminus of the protein translated from the coding region of the rat periostin gene obtained in Production Example 2 and a CMV promoter was prepared.
[0066] First, the vector fragment obtained by digesting the pTracer-CMV2 vector (Invitrogen) with restriction enzymes EcoRI and EcoRV was ligated with the ligation kit (Takara Shuzo Co., Ltd.) obtained by digesting the pTracer-CMV2 vector with restriction enzymes EcoRI and HindIII in Production Example 2. A fragment of about 500 bp obtained by rat periostin 5'RACE#1 and a fragment of about 2780 bp obtained by digesting rat periostin #1 obtained in Production Example 2 with restriction enzymes HindIII and Hpal, and the obtained plasmid was named pTracer - CMV2 / rat periostin. The pTracer-CMV2 / rat periostin thus prepared was digested with restrictio...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com