Method for coprocessing crystal violet polluted sewage by outdoor natural light-hydrogen peroxide
A technology of hydrogen peroxide and a treatment method is applied in the field of sewage treatment and treatment of crystal violet polluted sewage, and the effect of simple process, easy popularization and efficient utilization is achieved.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
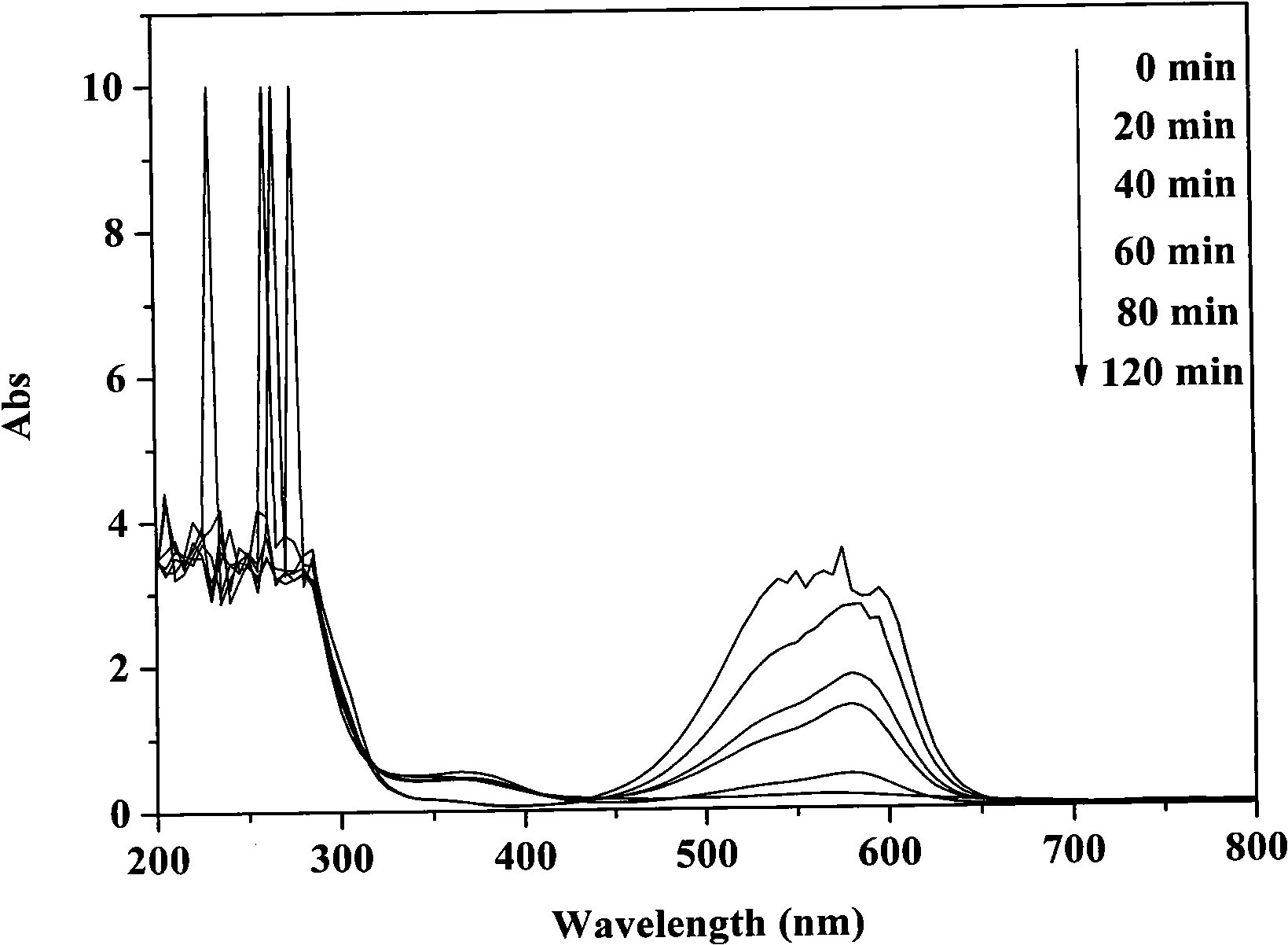
[0027] Disperse 0.1 g of bismuth silicate photocatalyst in 100 mL of crystal violet solution with an initial concentration of 40 mg / L, and ultrasonicate the mixture for 30 min under dark conditions to completely disperse the catalyst in the solution. Before irradiation, the mixture was magnetically stirred for 30 minutes in the dark, and after reaching adsorption equilibrium, 3 mL of hydrogen peroxide with a mass fraction of 30% was added, stirred and mixed evenly. The photocatalytic reactor was placed in the outdoor natural light and irradiated vertically, and the reaction was stirred for 2 h. After the reaction was completed, the photocatalyst was separated and recovered by suction filtration. During the reaction process, samples were taken every 20 min, and the supernatant was collected by centrifugation, and the concentration of crystal violet was analyzed and quantified with a UV-Vis spectrophotometer (Varian, cary50).
[0028] figure 1 is the change of crystal violet c...
Embodiment 2
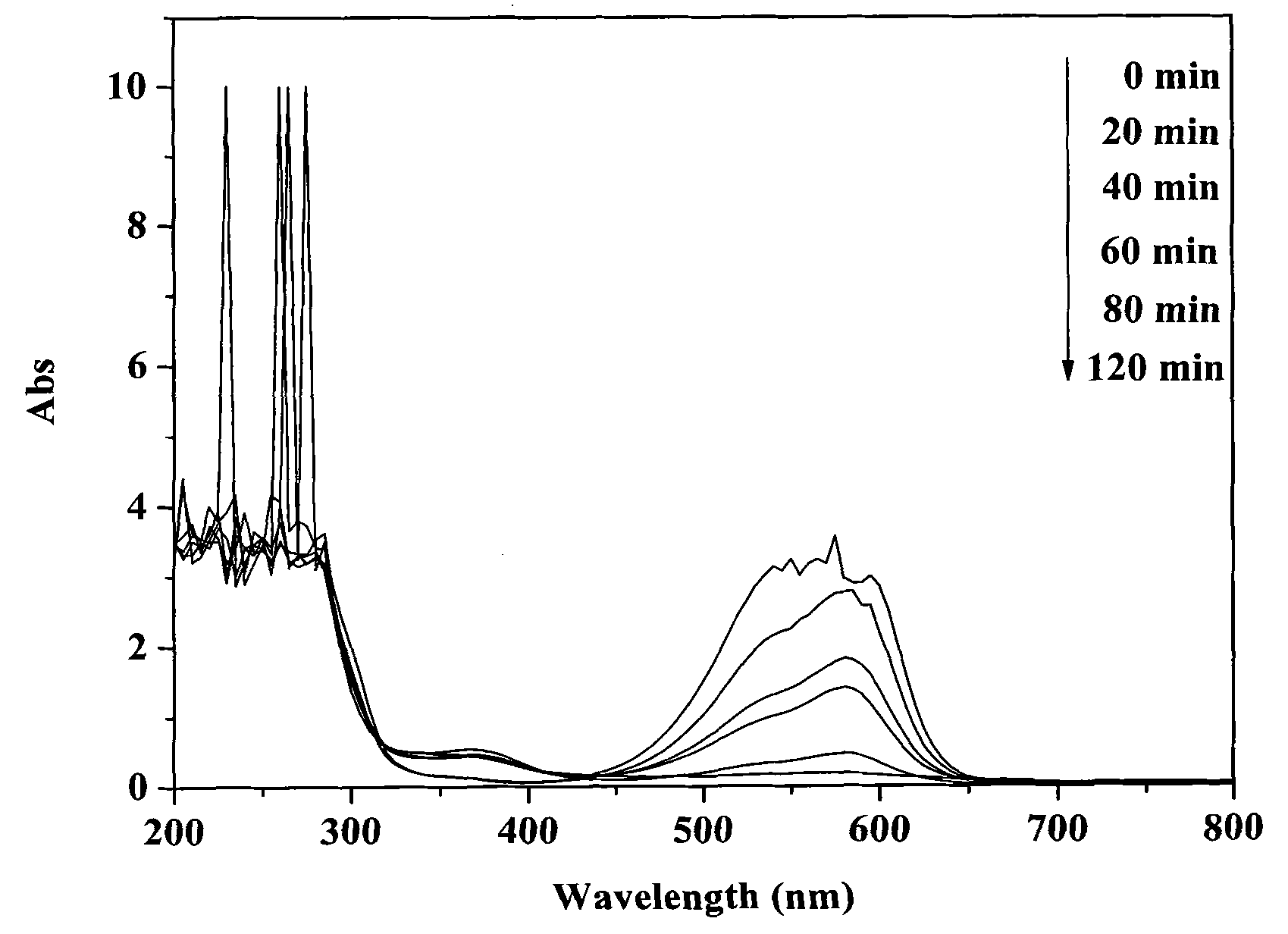
[0030] Disperse 0.1 g of bismuth silicate photocatalyst in 100 mL of crystal violet solution with an initial concentration of 30 mg / L, and ultrasonicate the mixture for 30 min under dark conditions to completely disperse the catalyst in the solution. Before irradiation, the mixture was magnetically stirred for 30 minutes in the dark, and after reaching adsorption equilibrium, 2 mL of 30% hydrogen peroxide was added, stirred and mixed evenly. The photocatalytic reactor was placed in the outdoor natural light and irradiated vertically, and the reaction was stirred for 2 h. After the reaction was completed, the photocatalyst was separated and recovered by suction filtration. During the reaction process, samples were taken every 20 min, and the supernatant was collected by centrifugation, and the concentration of crystal violet was analyzed and quantified with a UV-Vis spectrophotometer (Varian, cary50).
[0031] After 2 hours of reaction, the decolorization rate of crystal viole...
Embodiment 3
[0033] Disperse 0.1 g of bismuth silicate photocatalyst in 100 mL of crystal violet solution with an initial concentration of 30 mg / L, and ultrasonicate the mixture for 30 min under dark conditions to completely disperse the catalyst in the solution. Before irradiation, the mixture was magnetically stirred for 30 minutes in the dark, and after reaching adsorption equilibrium, 1.5 mL of 30% hydrogen peroxide was added, stirred and mixed evenly. The photocatalytic reactor was placed in the outdoor natural light and irradiated vertically, and the reaction was stirred for 2 h. After the reaction was completed, the photocatalyst was separated and recovered by suction filtration. During the reaction process, samples were taken every 20 min, and the supernatant was collected by centrifugation, and the concentration of crystal violet was analyzed and quantified with a UV-Vis spectrophotometer (Varian, cary50).
[0034] After 2 hours of reaction, the decolorization rate of crystal vio...
PUM
| Property | Measurement | Unit |
|---|---|---|
| quality score | aaaaa | aaaaa |
| decolorization rate | aaaaa | aaaaa |
| decolorization rate | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com