New compound and preparation method and application thereof
A compound and application technology, applied in the field of chemical synthesis, can solve problems such as complicated operation, and achieve the effect of less by-products and easy operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
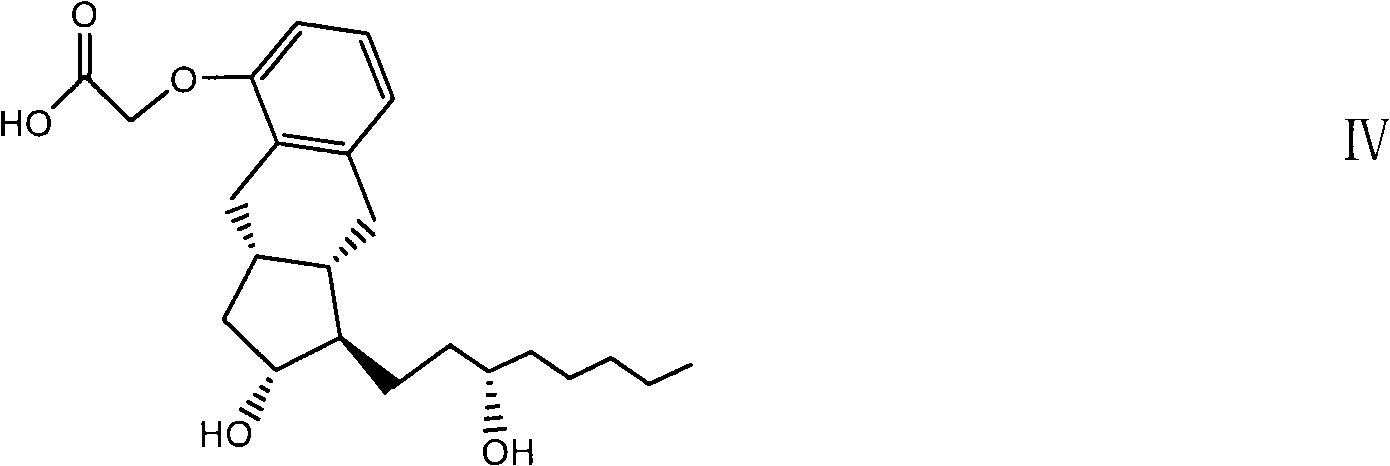
[0088] The prostacyclin UT15 obtained by the preparation method provided by the present invention can be used to prepare a drug for treating pulmonary hypertension, and the drug contains an effective amount of the prostacyclin UT15 and a pharmaceutically acceptable carrier.
[0089] As used herein, the terms "comprising" or "comprising" include "comprising", "consisting essentially of", and "consisting of".
[0090] As used herein, the term "consisting essentially of" means that in addition to essential ingredients or essential components, the composition may also contain a small amount of secondary ingredients and / or impurities that do not affect the active ingredients. For example, sweeteners to improve taste, antioxidants to prevent oxidation, and other additives commonly used in the art may be contained.
[0091] As used herein, the term "effective amount" refers to an amount that can produce functions or activities on humans and / or animals and that can be accepted by huma...
Embodiment 1
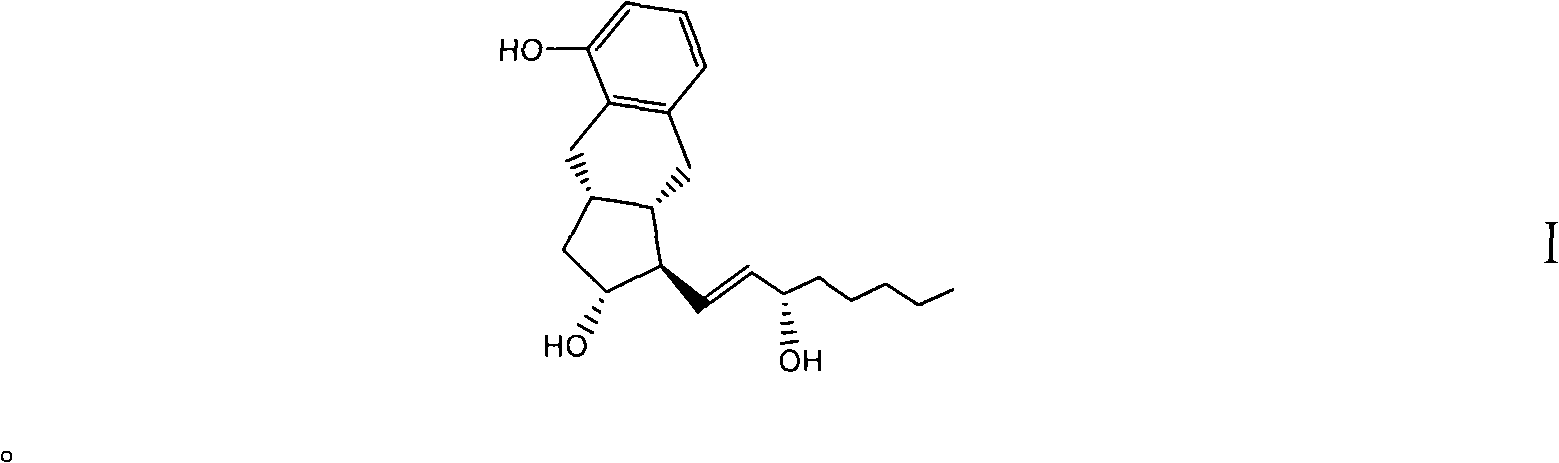
[0105] Preparation of compound I
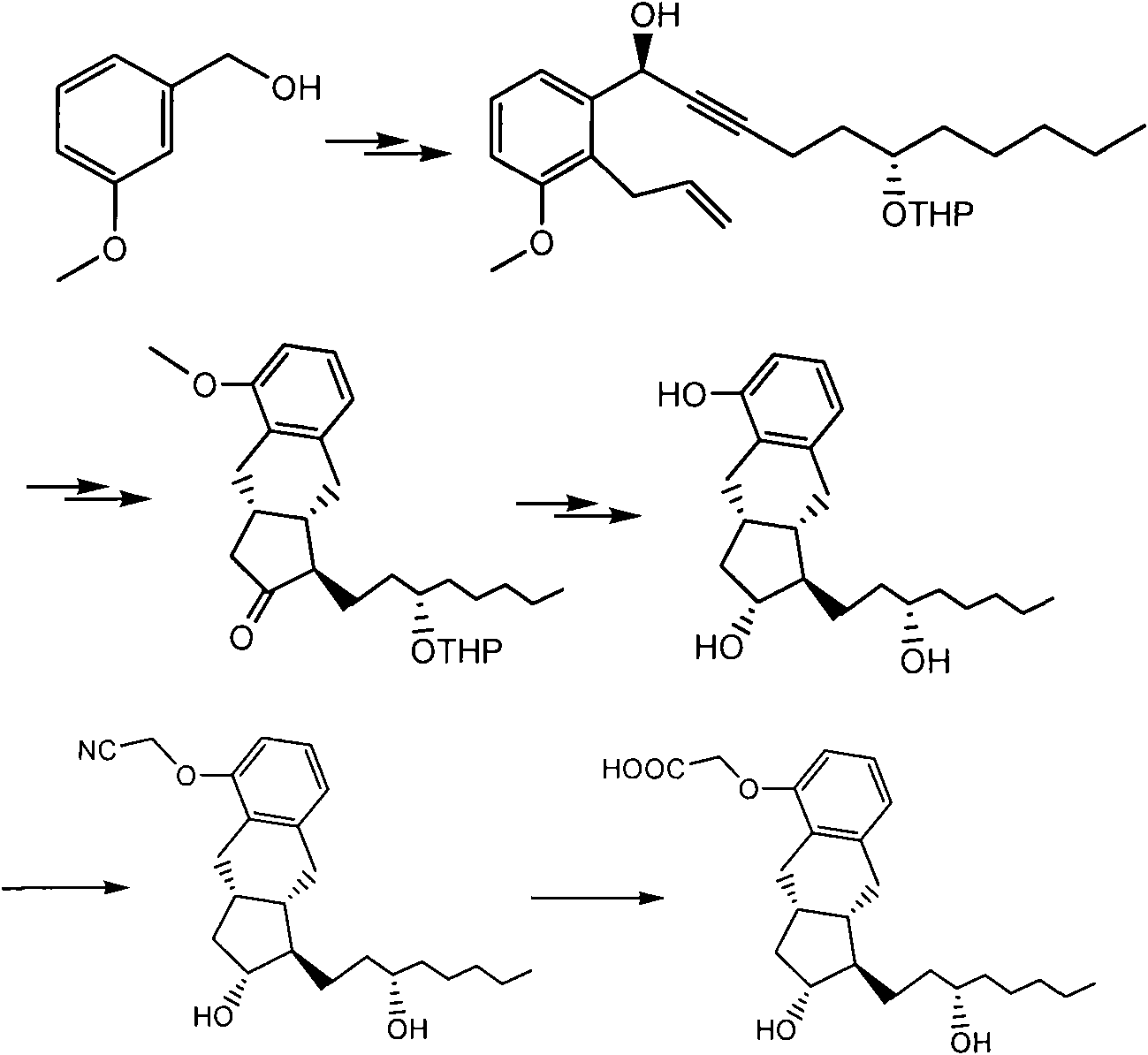
[0106] Compound II (2.80 g) was dissolved in 14 ml of tetrahydrofuran, then 21 ml of water and 42 ml of glacial acetic acid were added, and stirred to dissolve. N 2 Under protection, heated to 45°C and stirred for 4h. Slowly cool to room temperature, add semi-saturated brine (120ml), ethyl acetate (120ml) and stir to separate the layers, back-extract the aqueous layer with ethyl acetate (60ml), combine the ethyl acetate layers, and saturated sodium ), washed with saturated sodium chloride (100ml), dried over anhydrous sodium sulfate, and concentrated under reduced pressure to obtain compound 4 as a colorless oil, which was purified by silica gel column chromatography to obtain compound I (1.4g). m / z: 353.3(M+Na+)1H NMR (MeOD, 500MHz) δ0.89(t, 3H), 1.1-2.30(m, 17H), 2.41-2.45(m, 2H), 2.64-2.78(m, 2H), 3.45-3.54(m, 1H), 3.55-3.81(m, 1H), 5.67(m, 1H), 5.69(m, 1H), 6.65(d, 1H), 6.73(d, 1H), 6.99 (t, 1H); 13C NMRδ14.4, 23.8, 26.9, 29.9, 33.5, ...
Embodiment 2
[0108] Preparation of compound III
[0109] The compound I obtained in Example 1 was dissolved in ethyl acetate (56ml) in a 1L hydrogenation shaker flask, 10% palladium carbon (0.18g) was added, and the mixture was shaken at 20-25°C and hydrogen pressure of 50-60Psi for 8h. After adding a small amount of diatomaceous earth for filtration, the filtrate was concentrated to dryness under reduced pressure to obtain compound III (1.2 g). mp113-115°C; [a] 25 D +50.8 (c 0.324, MeOH).IR 3415, 3060, 2932, 753, and 702cm-1; m / z: 355.3 (M+Na + ); 1H NMR (MeOD, 500MHz) δ0.89(t, 3H), 1.1-2.30(m, 15H), 2.41-2.45(m, 2H), 2.64-2.78(m, 2H), 3.45-3.54(m, 1H ), 3.55-3.81(m, 1H), 5.66-5.70(m, 2H), 6.65(d, 1H), 6.73(d, 1H), 6.99(t, 1H);
[0110] 13 C NMR (MeOH, 125MHz) δ14.7, 24.0, 26.8, 26.9, 29.9, 33.5, 34.5, 34.9, 36.4, 38.6, 42.3, 42.7, 52.9, 73.2, 77.9, 114.1, 120.8, 126.4, 127.3, 142.2, 155.5 .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com