Preparation method of live vector vaccine for controlling chicken coccidiosis and application thereof
A live carrier vaccine and chicken coccidiosis technology, applied in the field of live carrier vaccine preparation, can solve problems such as unfavorable transportation and storage, cancer-causing DNA antibodies and gene drift, complex mechanism of action, etc., achieve simple fermentation and production technology, and be popularized and used The effect of value, prevention and treatment
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] Embodiment 1, the construction that contains antigenic gene 3-1E recombinant plasmid
[0040] 1. Cloning of antigen 3-1E gene
[0041] 1. Cloning of 3-1E gene
[0042] Purify sporulated oocysts from the feces of broiler chickens infected with E.tenella according to conventional techniques, and refer to the total RNA in pure sporulated oocysts, reverse transcribe the RNA into cDNA with a reverse transcription kit, and reverse The recorded cDNA was used as a template, and the upstream primer (SEQ ID NO: 1) was used:
[0043] 5P 31E-BS 5'-TCTAGAAATGGGTGAAGAGGCTGATACT-3' and
[0044] Downstream primer (SEQ ID NO: 2):
[0045] 3P 31E-BS 5'-CTGCAGTTA GTGATGGTGATGGTGATG GAAGCCGCCCTGGTACAGGT-3' was used for PCR to obtain the target gene (3-1E gene).
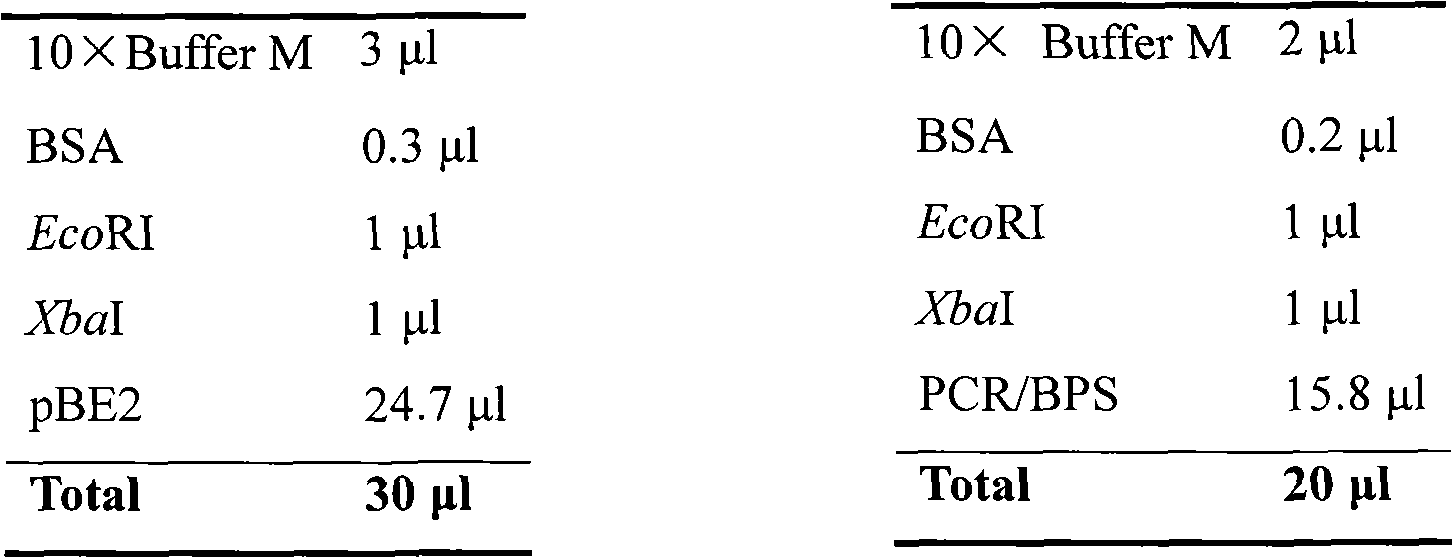
[0046] The PCR reaction system is:
[0047] cDNA 1.0μl
[0048] Primer 5P 31E-BS (10mM) 1.0μl
[0049] Primer 3P 31E-BS (10mM) 1.0μl
[0050] Taq enzyme (5U / μl) 0.5μl
[0051] 10×PCR buffer (with Mg 2+ ) 5.0μl
...
Embodiment 2
[0080] Embodiment 2, the preparation method of recombinant bacillus subtilis (the live carrier vaccine of chicken coccidiosis prevention):
[0081] Transform the recombinant expression vector (recombinant plasmid) pBES-3-1E constructed in Example 1 above into Bacillus subtilis 1A751 (derived from the American Bacillus Collection Center BGSC) (any Bacillus subtilis strain can be transformed):
[0082] Bacillus subtilis 1A751 was cultured overnight at 30°C in 4ml of GMI medium (test tube), 100r / min (slow shaking) 10% (volume ratio) was transferred to 6ml of GMI medium (erlenmeyer flask), and cultivated at 37°C for 3.5 h, 200r / min (quick shaking); 10% was transferred to 20ml GMII medium (erlenmeyer flask), cultured at 37°C for 90min, 100r / min (slow shaking); the bacteria were collected by centrifugation (1 / 10 volume supernatant Suspended bacteria): Divide 20ml of bacterial liquid into four 10ml centrifuge tubes (5ml each), collect the bacterial cells by centrifugation, and resusp...
Embodiment 3
[0085] Embodiment 3, the preparation of the oral biological preparation of prevention chicken coccidiosis
[0086] Mix 25 parts by weight of zeolite powder with 75 parts by weight of cornstarch, and both the zeolite powder and cornstarch can pass through a 100-mesh sieve; the carrier is obtained.
[0087] The bacteria powder obtained in Example 2 is mixed with the above-mentioned carrier in a weight ratio of 1:9, and simply stirred and mixed to obtain an oral biological agent for preventing and treating chicken coccidiosis; each gram of the oral biological agent contains 10 9 cfu live vector vaccine.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com