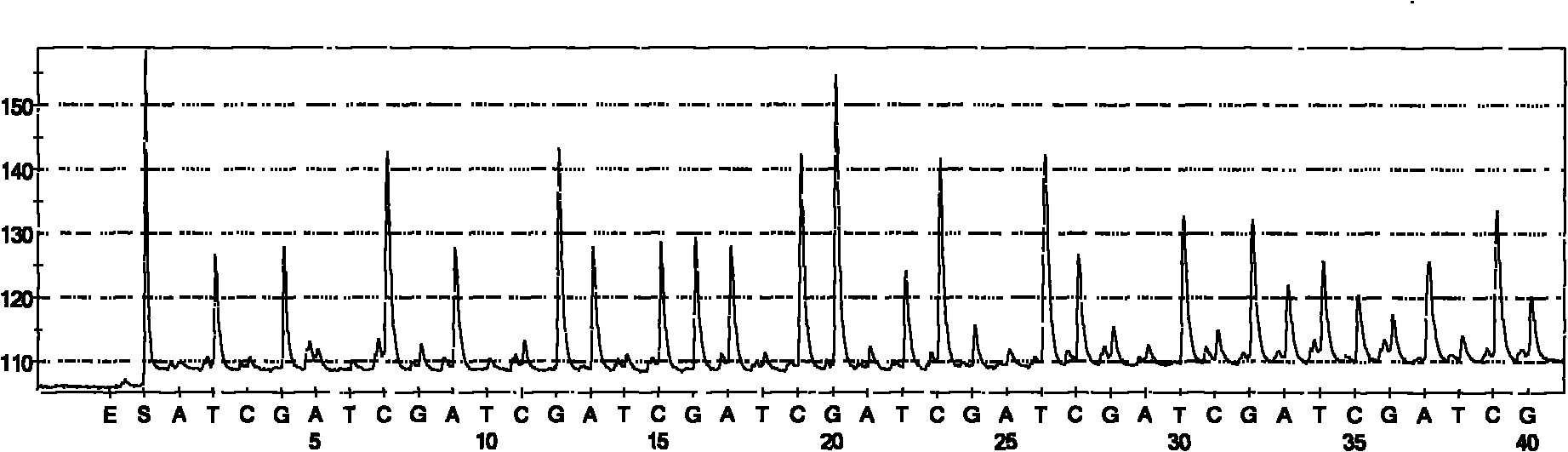
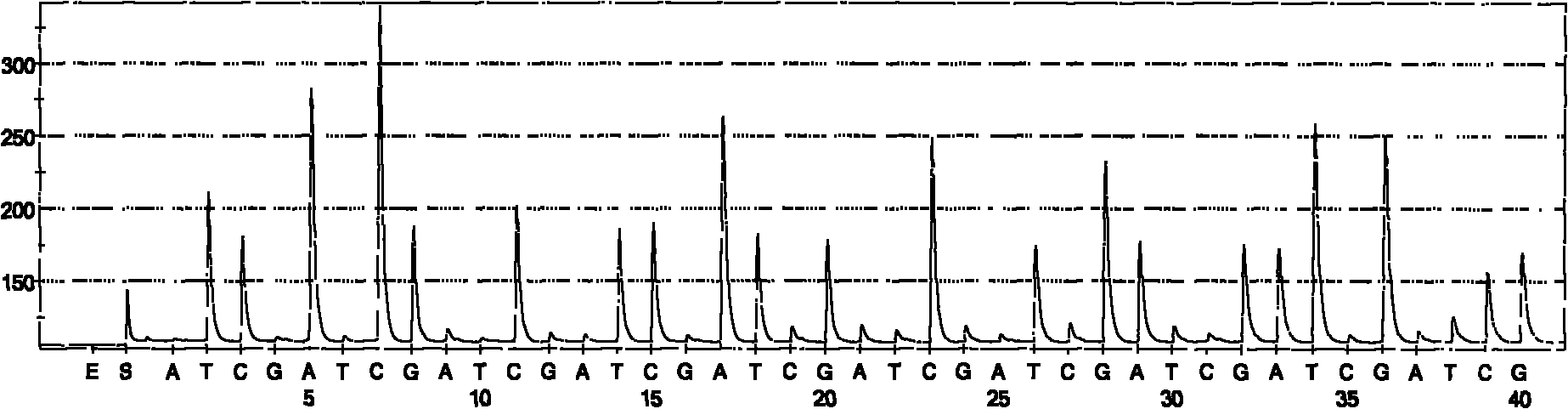
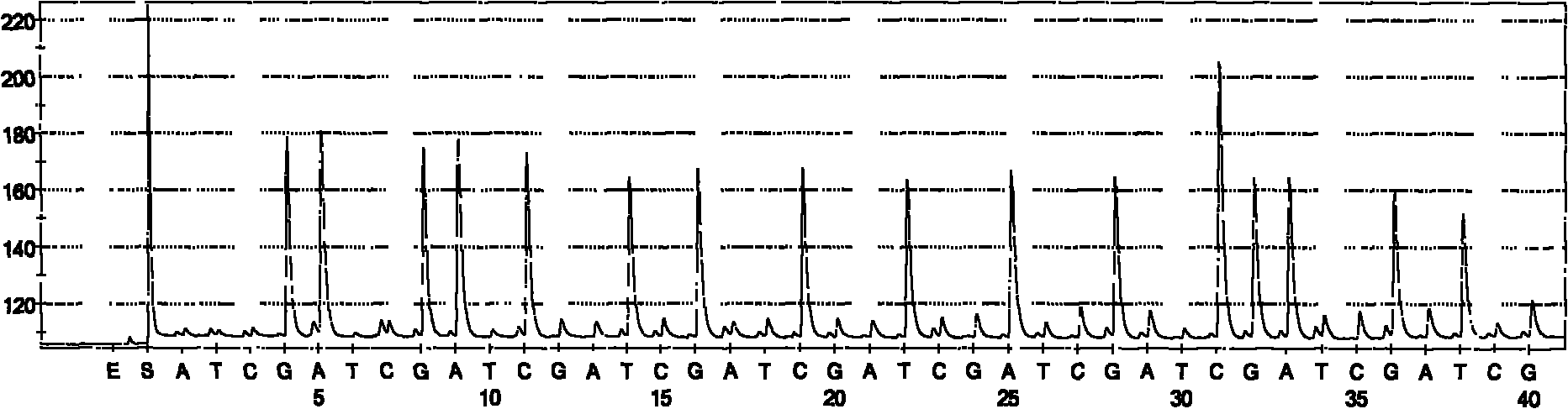
Hepatitis C sequencing and typing kit and detection method thereof
A detection method and kit technology, which is applied in the field of hepatitis C sequencing typing kit and its detection, can solve the problems of poor stability and low accuracy, and achieve high accuracy, low cost, and high throughput
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0061] Example 1: Preparation of the kit.
[0062] (1) RNA extraction reagent:
[0063] Trizol: purchased from Invitrogen,
[0064] Chloroform: purchased from Hangzhou Gaojing Fine Chemical Co., Ltd.
[0065] Isopropanol: purchased from Hangzhou Shuanglin Chemical Reagent Factory,
[0066] Absolute ethanol: purchased from Hangzhou Changzheng Chemical Reagent Co., Ltd.
[0067] DEPC treated water: purchased from Fermentas Company.
[0068] (2) Reagents for reverse transcription:
[0069] 200U / μL M-MLV reverse transcriptase: purchased from Fermentas Company,
[0070] 20U / μL RNase inhibitor: purchased from Fermentas Company,
[0071] 5×RT-PCR Buffer: 250mM Tris-HCl (pH 8.3), 250mM KCl, 20mM MgCl 2 , 50mMDTT (purchased from fermentas company),
[0072] 10mM dNTP mix: purchased from Shanghai Dingguo Biotechnology Co., Ltd.,
[0073] 100 μM random hexamer primer: purchased from Invitrogen Company,
[0074] DEPC treated water: purchased from Fermentas Company.
[0075] (3)...
Embodiment 2
[0092] Embodiment 2: detection method.
[0093] (1) The extraction of hepatitis C HCV RNA template specifically includes the following steps:
[0094] (1a) Mix 200 μL of sample serum with 1 mL Trizol, add 200 μL of chloroform, mix for 15 seconds, room temperature for 5 minutes, and centrifuge at 12000 g for 15 minutes at 4°C (the centrifuge is Microfuge 22R Centrinofuge, BECKMAN Company, the same below);
[0095] (1b) Transfer the upper aqueous phase into another centrifuge tube, add isopropanol equal to the volume of the upper aqueous, mix for 15 seconds, room temperature for 10 minutes, and centrifuge at 12000g for 10 minutes at 4°C;
[0096] (1c) Discard the supernatant obtained in step (1b), add ice-precooled 75% (v / v) ethanol (mixed with DEPC water) 1mL, and centrifuge at 7500g for 5min at 4°C;
[0097] (1d) Discard the supernatant obtained in step (1c), air-dry for 5 min, add 20 uLDEPC-treated water, and obtain HCV RNA at -70°C for later use.
[0098] (2) the RNA templ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com