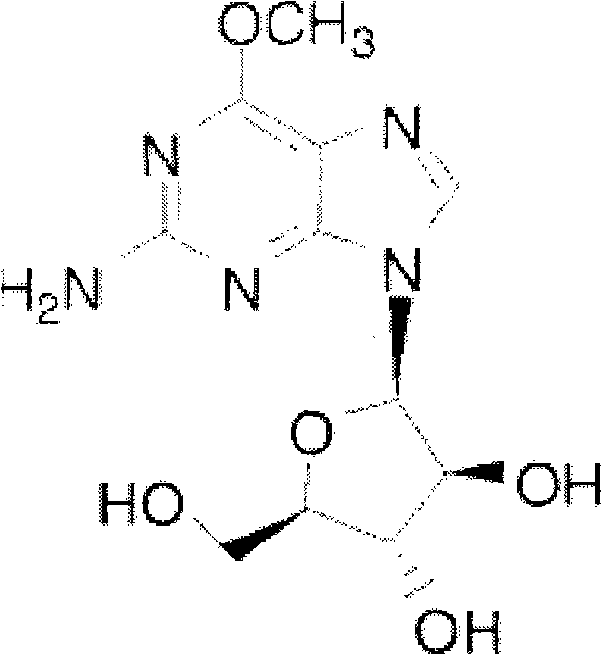
Intravenous injection of nelarabine and preparation method thereof
A technology for osmotic pressure regulators and injections, which is applied in the field of nelarabine infusion preparations and injections, can solve the problems of reducing the dosage of the main drug, and achieve the effect of reducing adsorption and removing pyrogens
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
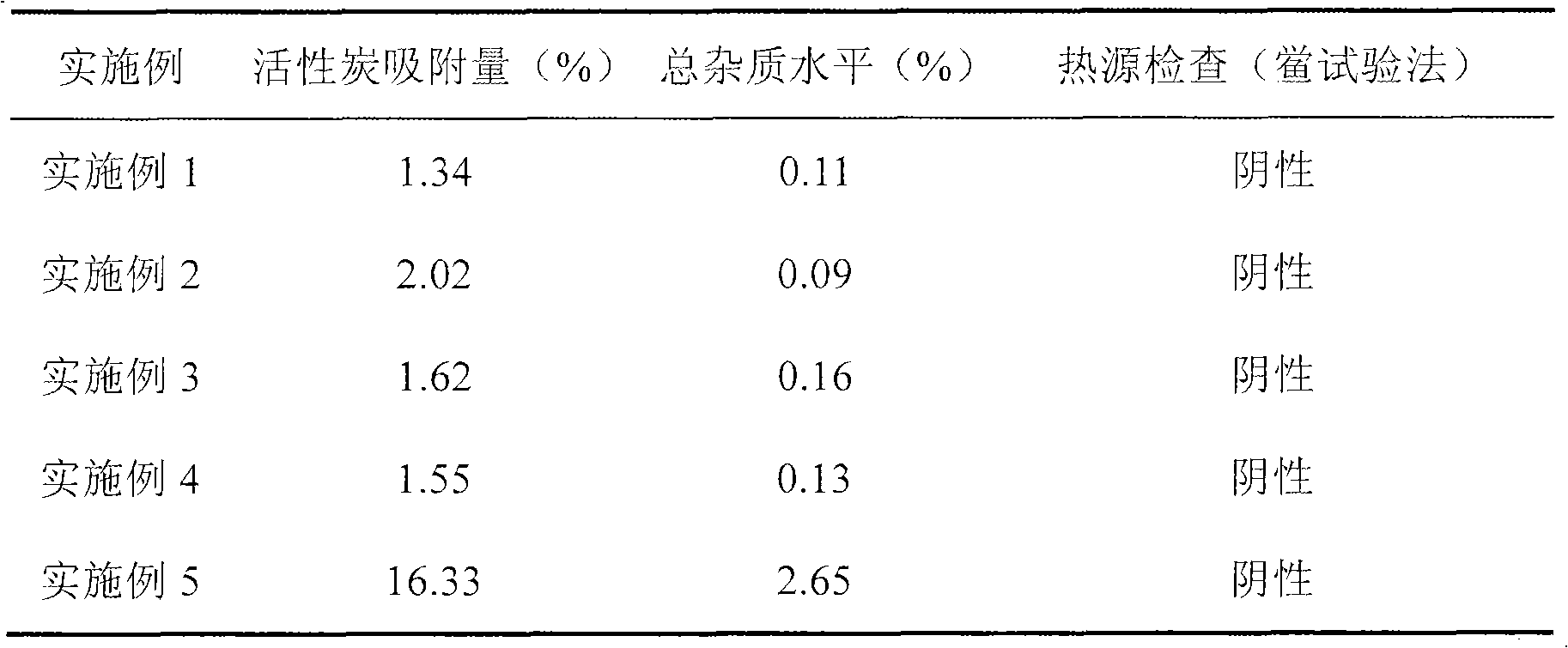
Examples
Embodiment 1
[0025] Prescription composition:
[0026] Nalarabine 250g
[0028] Propylene glycol 50.0g
[0029] Add water for injection to 50L
[0030]
[0031] Made 1000 pieces
[0032] Mix propylene glycol for injection with 80% water for injection, add nelarabine and stir to dissolve; add hydrochloric acid / sodium hydroxide to adjust the pH value to 5.0-7.0, add an appropriate amount of activated carbon for needles, stir, filter and decarbonize, add water for injection to The whole amount is finely filtered through a microporous membrane, potted, and autoclaved at 121°C for 15 minutes to obtain the finished product.
Embodiment 2
[0034] Prescription composition:
[0035] Nalarabine 250g
[0037] Propylene glycol 25.0g
[0038] Add water for injection to 50L
[0039]
[0040] Made 1000 pieces
[0041] Mix propylene glycol for injection with 80% water for injection, add nelarabine and stir to dissolve; add hydrochloric acid / sodium hydroxide to adjust the pH value to 5.0-7.0, add an appropriate amount of activated carbon for needles, stir, filter and decarbonize, add water for injection to The whole amount is finely filtered through a microporous membrane, potted, and autoclaved at 121°C for 15 minutes to obtain the finished product.
Embodiment 3
[0043] Prescription composition:
[0044] Nalarabine 250g
[0046] Propylene glycol 2.50g
[0047] Add water for injection to 50L
[0048]
[0049] Made 1000 pieces
[0050] Mix propylene glycol for injection with 80% water for injection, add nelarabine and stir to dissolve; add hydrochloric acid / sodium hydroxide to adjust the pH value to 5.0-7.0, add an appropriate amount of activated carbon for needles, stir, filter and decarbonize, add water for injection to The whole amount is finely filtered through a microporous membrane, potted, and autoclaved at 121°C for 15 minutes to obtain the finished product.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com