Method for preparing scutellarin
A technology of acetylation and compounds, applied in the field of drug synthesis, can solve the problems of low yield, lack of raw material sources, cumbersome process, etc., and achieve the effect of high yield, rich source, and simple process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Example Embodiment
Example 1:
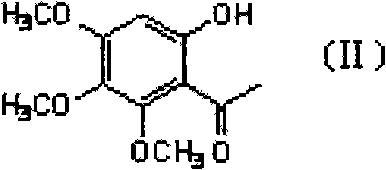
Step 1: Preparation of compound II
Weigh 18.4g (0.1mol) 3,4,5-trimethoxyphenol into the reaction vessel, add about 30ml acetic anhydride, and slowly add BF while stirring at room temperature 3 The solution was 10ml, and then slowly heated to 40°C to react for 3 hours (TLC checked that the raw material had reacted completely).
The above reaction solution was naturally cooled slightly, 100 ml of ethyl acetate was added and stirred, and then placed in the refrigerator overnight, and filtered to obtain a yellow solid precipitated. Add 100 ml of water and 10 ml of ethanolamine to the yellow solid, stir thoroughly for 1 to 2 hours, and then extract the product with ethyl acetate twice, each with 80 ml. The extracts were combined, washed once with water, dried over anhydrous sodium sulfate, filtered, and evaporated to remove the solvent under reduced pressure to obtain compound II as a light yellow oil, which can be solidified after being refrigerated and dried and weighed...
Example Embodiment
Example 2:
Step 1: Preparation of compound II
Weigh 18.4g (0.1mol) 3,4,5-trimethoxyphenol into the reaction vessel, add about 30ml acetic acid, slowly add 50g polyphosphoric acid under stirring at room temperature, and then slowly heat to 80°C for 1 hour (TLC Check that the raw materials have reacted completely).
Under stirring, the above reaction solution was poured into 150 ml of ice-cold water while it was hot, and then the product was extracted twice with 100 ml of ethyl acetate. The extracts were combined, washed once with water, dried over anhydrous sodium sulfate, filtered, and evaporated to remove the solvent under reduced pressure to obtain compound II as a light yellow oil, which can be solidified after being refrigerated and dried and weighed. The yield: 85%. 1 HNMR(CDCl 3 ):Cit.
Step 2: Preparation of compound III
Weigh 15.2g (0.10mol) 3,4,5-trimethoxybenzoic acid into the reaction flask, add 40ml dichloromethane, 15ml SOCl 2 (Thionyl chloride) and 2~5 drops of D...
Example Embodiment
Example 3:
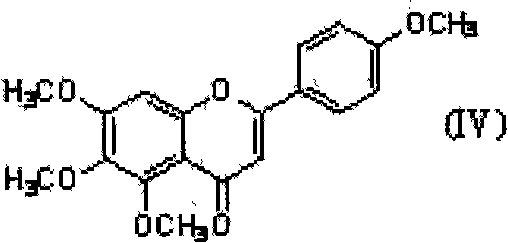
Step 1: Preparation of compound II
Weigh 4.0 g (0.011 mol) of Compound IV into the reaction flask, and add 50 ml of hydroiodic acid acetic acid solution. After mixing, heat to 100°C and react for 15 hours under nitrogen protection (TLC checks that the raw materials have reacted completely).
Under stirring, the above reaction solution was poured into 150 ml of ice-cold water while it was hot, and then the product was extracted twice with 100 ml of ethyl acetate. The extracts were combined, washed once with water, dried over anhydrous sodium sulfate, filtered, and evaporated to remove the solvent under reduced pressure to obtain compound II as a light yellow oil, which can be solidified after being refrigerated and dried and weighed. The yield: 85%. 1 HNMR(CDCl 3 ):Cit.
Step 2: Preparation of compound III
Weigh 15.2g (0.10mol) 3,4,5-trimethoxybenzoic acid into the reaction flask, add 40ml dichloromethane, 15ml SOCl 2 (Thionyl chloride) and 2~5 drops of DMF (N,N~dime...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com