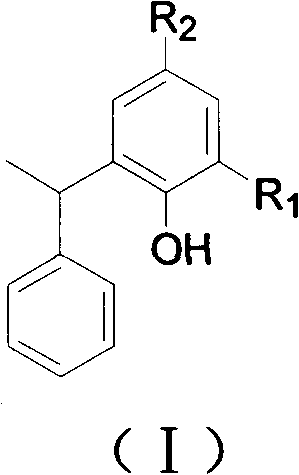
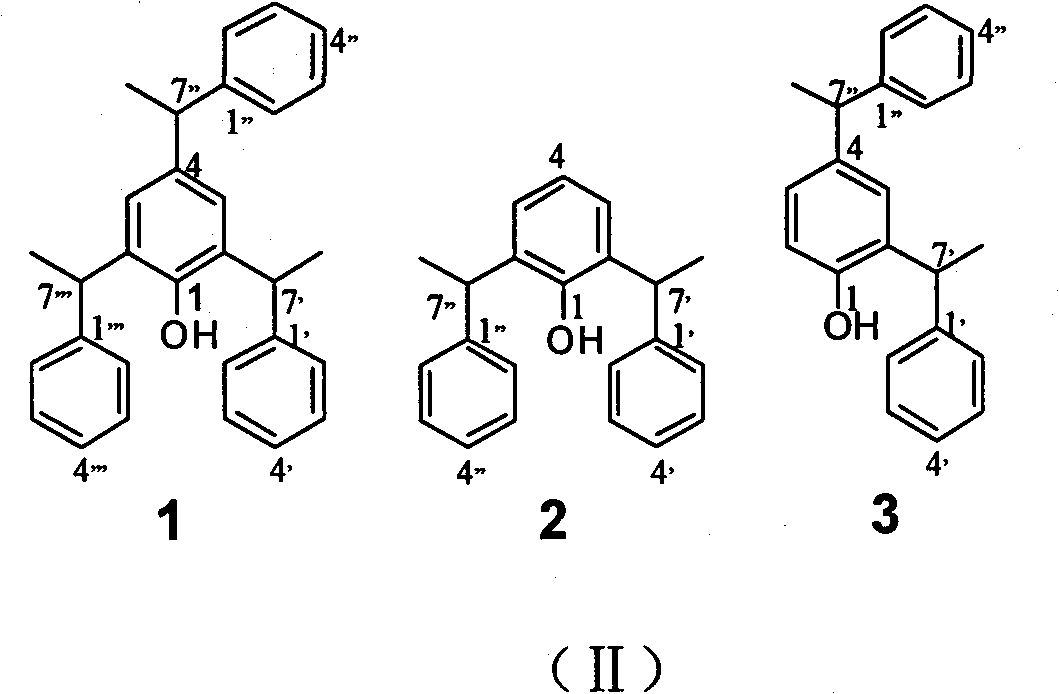
Application of styrene phenol compounds to preparing insulin sensitizer
A technology of phenolic compounds and insulin sensitizers, applied in the field of microbial drugs, can solve problems that are difficult to become drugs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028]Streptomyces deep-sea strain 220225 was isolated from the deep-sea mud of the South China Sea (13°24.987′N, 110°9.202′E) using Gaul’s medium. No characteristic sugars. The optimum NaCl concentration for growth is 1% by mass, and the highest tolerable NaCl concentration is 25% by mass. The GenBank accession number of the 16SrRNA gene sequence of this bacterial strain is FJ429837, and its sequence is:
[0029] GCGTGGGCGT GCTTACCATG CAAGTCGAAC GATGAACCAC TTCGGTGGGG ATTAGTGGCG
[0030] AACGGGTGAG TAACACGTGG GCAATCTGCC CTGCACTCTG GGACAAGCCC TGGAAACGGG
[0031] GTCTAATACC GGATACTGAT CATCTTGGGC ATCCTTGGTG ATCGAAAGCT CCGGCGGTGC
[0032] AGGATGAGCC CGCGGCCTAT CAGCTTGTTG GTGAGGTAAT GGCTCACCAA GGCGACGACG
[0033] GGTAGCCGGC CTGAGAGGGC GACCGGCCAC ACTGGGACTG AGACACGGCC CAGACTCCTA
[0034] CGGGAGGCAG CAGTGGGGAA TATTGCACAA TGGGCGAAAG CCTGATGCAG CGACGCCGCG
[0035] TGAGGGATGA CGGCCTTCGG GTTGTAAACC TCTTTCAGCA GGGAAGAAGC GAAAGTGACG
[0036] GTACCTGCAG AAGAAGCGCC GGCTAACTAC GTGCCAGC...
Embodiment 2
[0054] Example 2: Large-scale fermentation of bacterial strain 220225 and its fermentation product sample pretreatment method
[0055] Streptomyces deep-sea 220225 was activated on a slope, inoculated in FM3 medium, 28°C, 220r min -1 Shake culture for 3 days, inoculate into 30L FM3 medium according to 5% inoculum size, 28°C, 220r min -1 Shaking culture was carried out for 7 days to obtain the fermented product. The fermented product was concentrated under reduced pressure at 60°C to an extract, added an appropriate amount of methanol (3 L) for extraction, filtered off the extract (repeated twice), and concentrated to dryness to obtain a crude extract. The FM3 medium is composed of the following components by weight to volume: soluble starch 20g / L, yeast powder 5g / L, soybean powder 15g / L, peptone 2g / L, calcium carbonate 4g / L, sea salt 18g / L, The pH was adjusted to 7.0 (Garcia, G.D.; Romero, M.F.; Perez, B.J.; Garcia, D.T. Thiodepsipeptide isolated from a marine actinomycete W...
Embodiment 3
[0056] Embodiment 3: the separation of compound
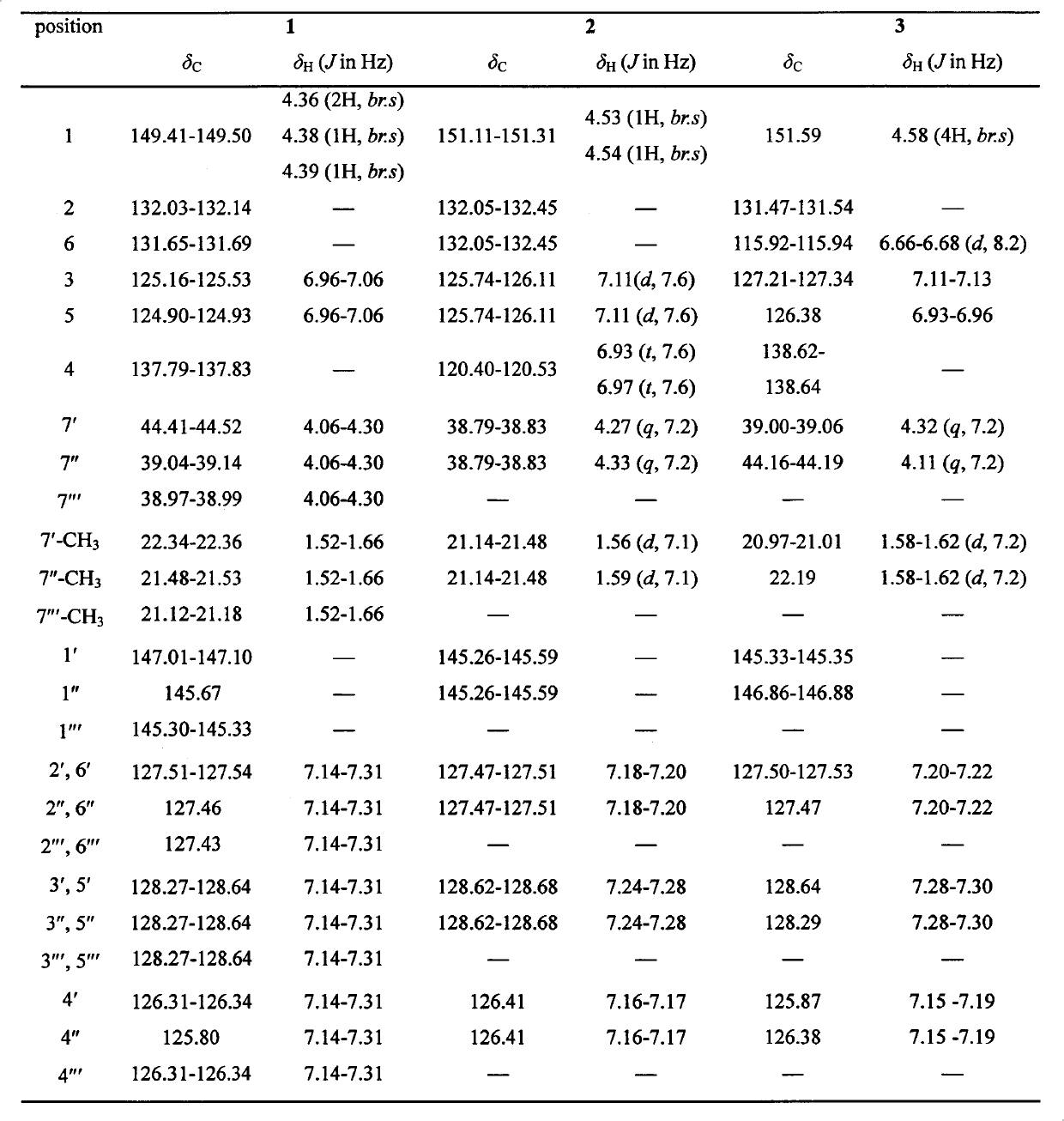
[0057] The crude extract obtained in Example 2 is suspended with methanol aqueous solution of 90% by volume (the mass volume ratio of the crude extract and methanol aqueous solution is 10g / L), and then with equal volumes of cyclohexane, chloroform and Extract with ethyl acetate to obtain cyclohexane extract layer (A), chloroform extract layer (B), ethyl acetate extract layer (C) and water layer (D). The chloroform extraction layer (B) was subjected to silica gel column chromatography, and cyclohexane-ethyl acetate gradient elution (100:0, 98:2, 9:1, 8:2, 7:3, 0:100, MeOH) , 7 subfractions of 220225B-A1, 220225B-A2, 220225B-A3, 220225B-A4, 220225B-A5, 220225B-A6 and 220225B-A7 were obtained. Fraction 220225B-A2 (656.5 mg) and 220225B-A3 (133.1 mg) were separated by Sephadex-LH 20 column chromatography and reverse phase C18 high performance liquid chromatography (HPLC) to obtain compound 1 (8.1 mg), 2 ( 8.5 mg), compound 3 (3.5...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com