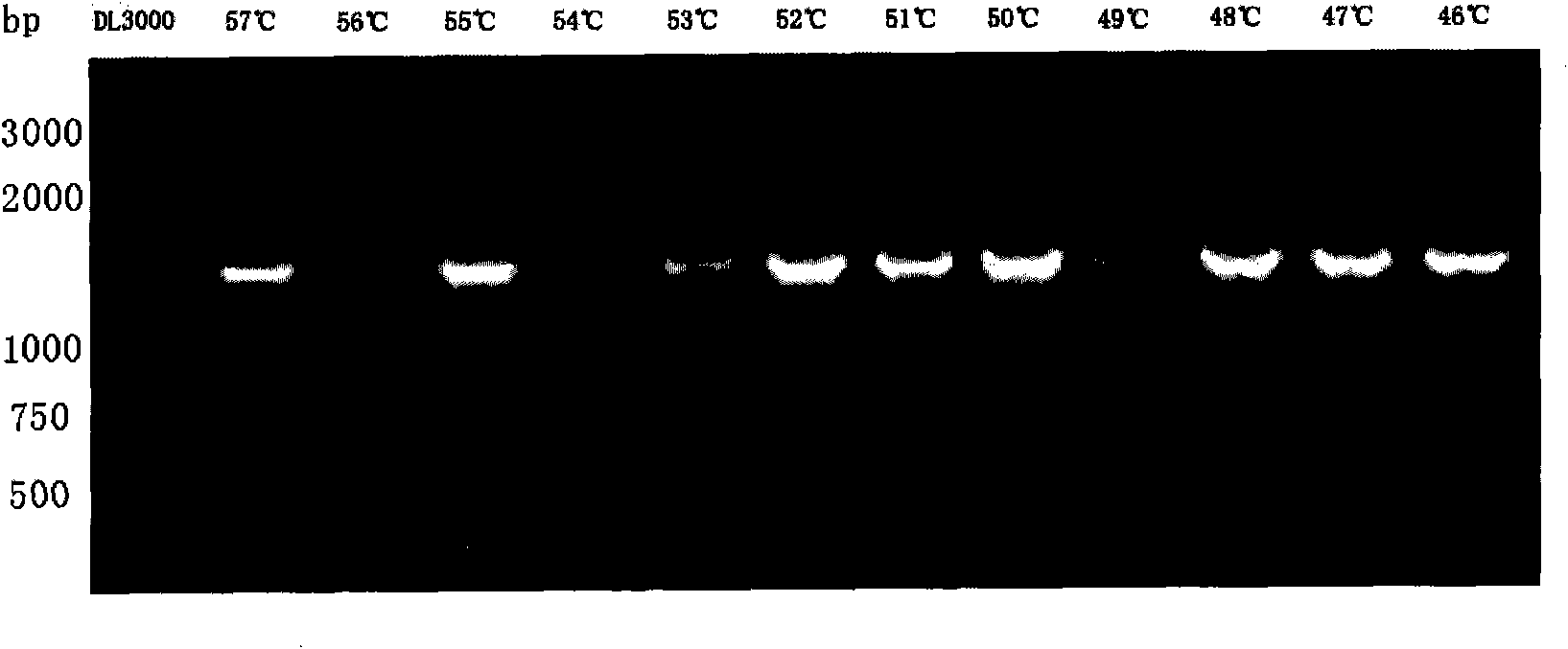
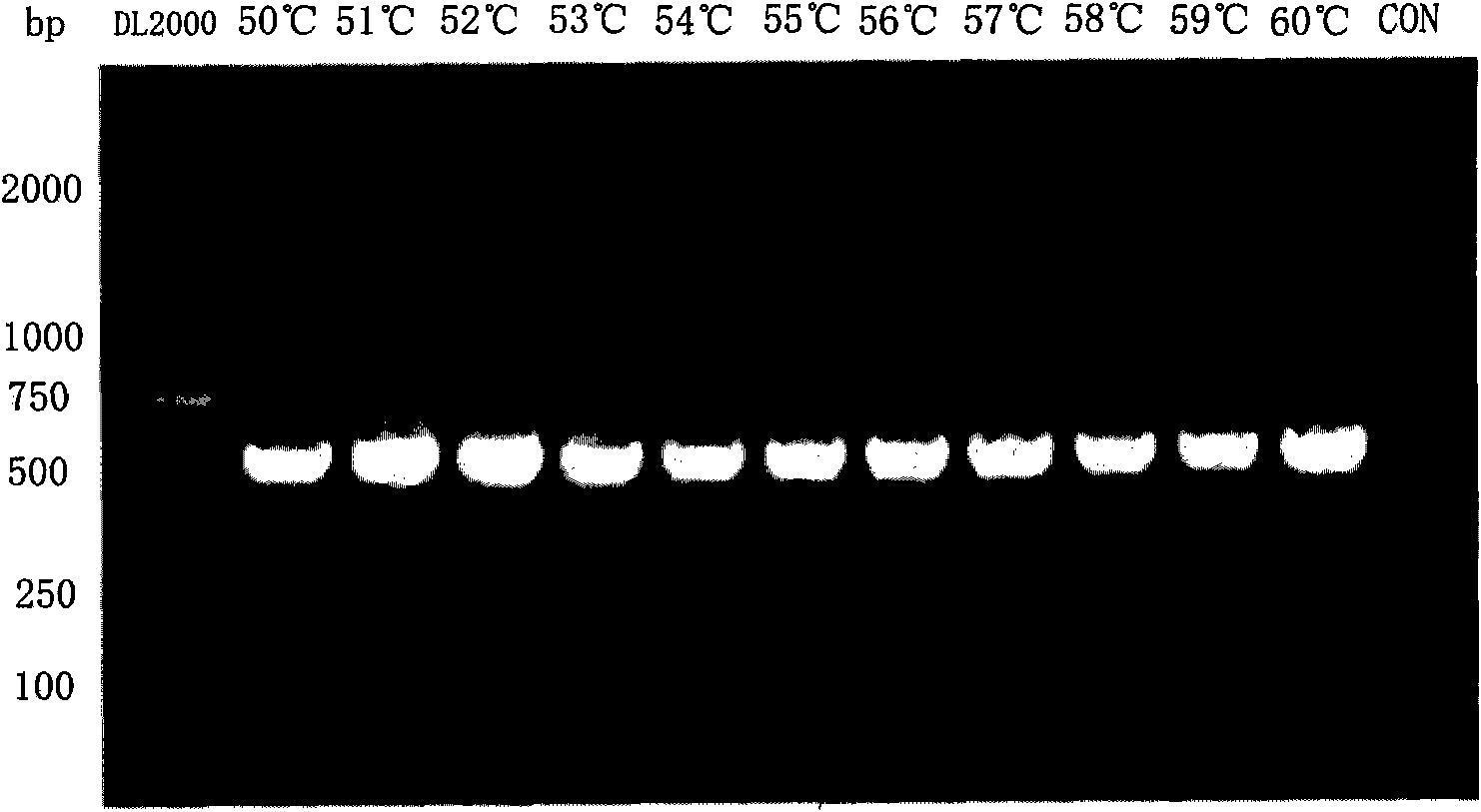
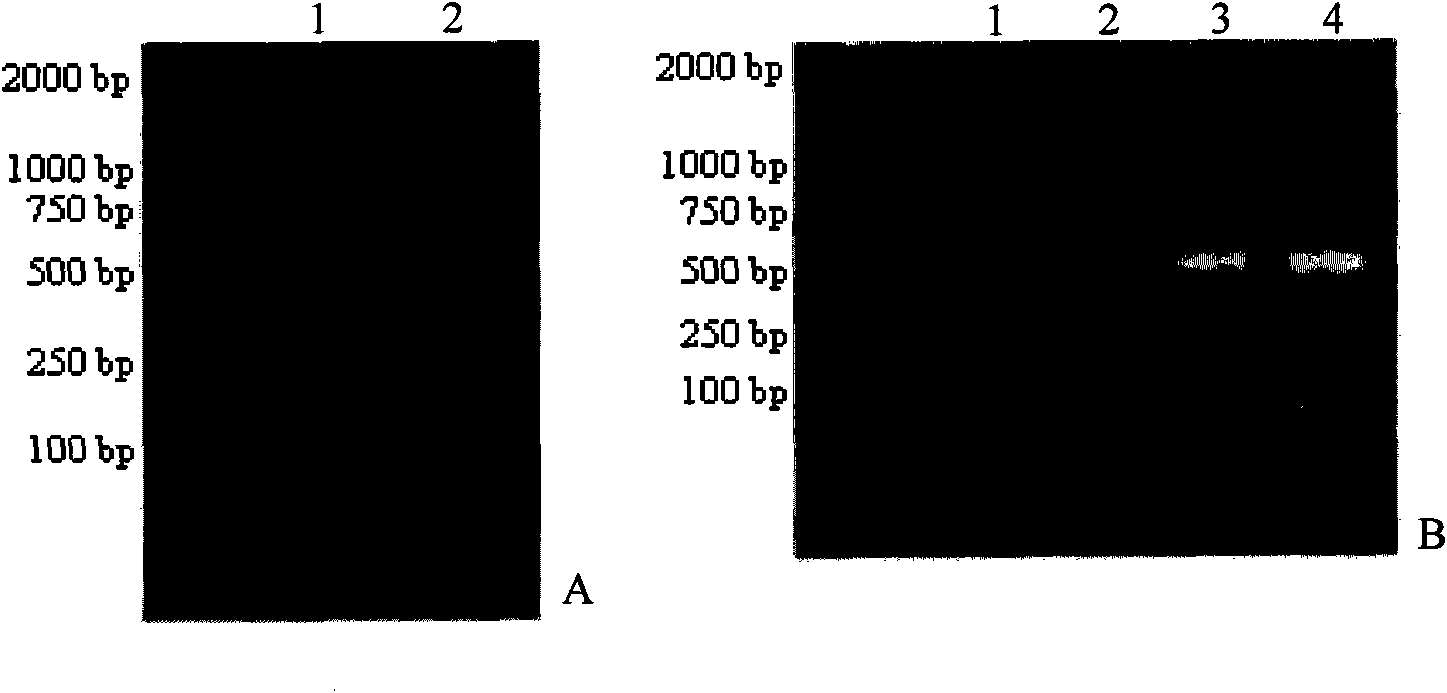
Detection method of infectious spleen and kidney necrosis virus Nest-PCR and kit thereof
A technology of spleen-kidney necrosis virus and detection method, which is applied in the detection field of infectious spleen-kidney necrosis virus Nest-PCR, can solve the problems of low specificity and low detection sensitivity, achieve high sensitivity, strong specificity, and shorten detection time Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] 1) 100mg of spleen and kidney tissues of infected and dying mandarin fish were suspended in 1ml of 1×PBS, and homogenized on ice.
[0033] 2) Centrifuge at 8000 rpm for 10 minutes at low temperature.
[0034] 3) Put the supernatant into a centrifuge tube, add 500 μl Tris saturated phenol and 500 μl chloroform:isoamyl alcohol (24:1). Mix well.
[0035] 4) 12000rpm, 10min.
[0036] 5) Aspirate the supernatant. Be careful not to aspirate the middle and lower layers to avoid mixing in protein contamination.
[0037] 6) Add one-tenth volume of NaAc, 1ml of absolute ethanol, mix well, and store at -20°C for 30min.
[0038] 7) 12000rpm, 10min.
[0039] 8) The supernatant was discarded, and the precipitate was washed twice with 70% ethanol, each time at 12000 rpm for 5 min.
[0040] 9) At room temperature, air-dry until semi-dry, and dissolve in 50 μl of water.
[0041] 10) Store at -20°C for later use.
Embodiment 2
[0043] 1) 1ml GF cell suspension with complete CPE, freeze-thawed 3 times.
[0044] 2) Centrifuge at 8000 rpm for 10 minutes at low temperature.
[0045] 3) Put the supernatant into a centrifuge tube, add 500 μl Tris saturated phenol and 500 μl chloroform:isoamyl alcohol (24:1). Mix well.
[0046] 4) 12000rpm, 10min.
[0047] 5) Aspirate the supernatant. Be careful not to aspirate the middle and lower layers to avoid mixing in protein contamination.
[0048] 6) Add one-tenth volume of NaAc, 1ml of absolute ethanol, mix well, and store at -20°C for 30min.
[0049] 7) 12000rpm, 10min.
[0050]8) The supernatant was discarded, and the precipitate was washed twice with 70% ethanol, each time at 12000 rpm for 5 min.
[0051] 9) At room temperature, air-dry until semi-dry, and dissolve in 50 μl of water.
[0052] 10) Store at -20°C for later use.
Embodiment 3
[0054] 1) 100mg of spleen and kidney tissue of infected and dying American redfish, suspended in 1ml 1×PBS, and homogenized on ice.
[0055] 2) Centrifuge at 8000 rpm for 10 minutes at low temperature.
[0056] 3) Put the supernatant into a centrifuge tube, add 500 μl Tris saturated phenol and 500 μl chloroform:isoamyl alcohol (24:1). Mix well.
[0057] 4) 12000rpm, 10min.
[0058] 5) Aspirate the supernatant. Be careful not to aspirate the middle and lower layers to avoid mixing in protein contamination.
[0059] 6) Add one-tenth volume of NaAc, 1ml of absolute ethanol, mix well, and store at -20°C for 30min.
[0060] 7) 12000rpm, 10min.
[0061] 8) The supernatant was discarded, and the precipitate was washed twice with 70% ethanol, each time at 12000 rpm for 5 min.
[0062] 9) At room temperature, air-dry until semi-dry, and dissolve in 50 μl of water.
[0063] 10) Store at -20°C for later use.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com