Method for improving detection accuracy of electrochemical active metal ions
A metal ion and detection method technology, which is applied in the field of improving the detection accuracy of electrochemically active metal ions, can solve the problems of large repeatability, difficult to achieve high consistency, and long processing time, so as to speed up the analysis speed. , The effect of reducing the loss of reagents and the number of analyses
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
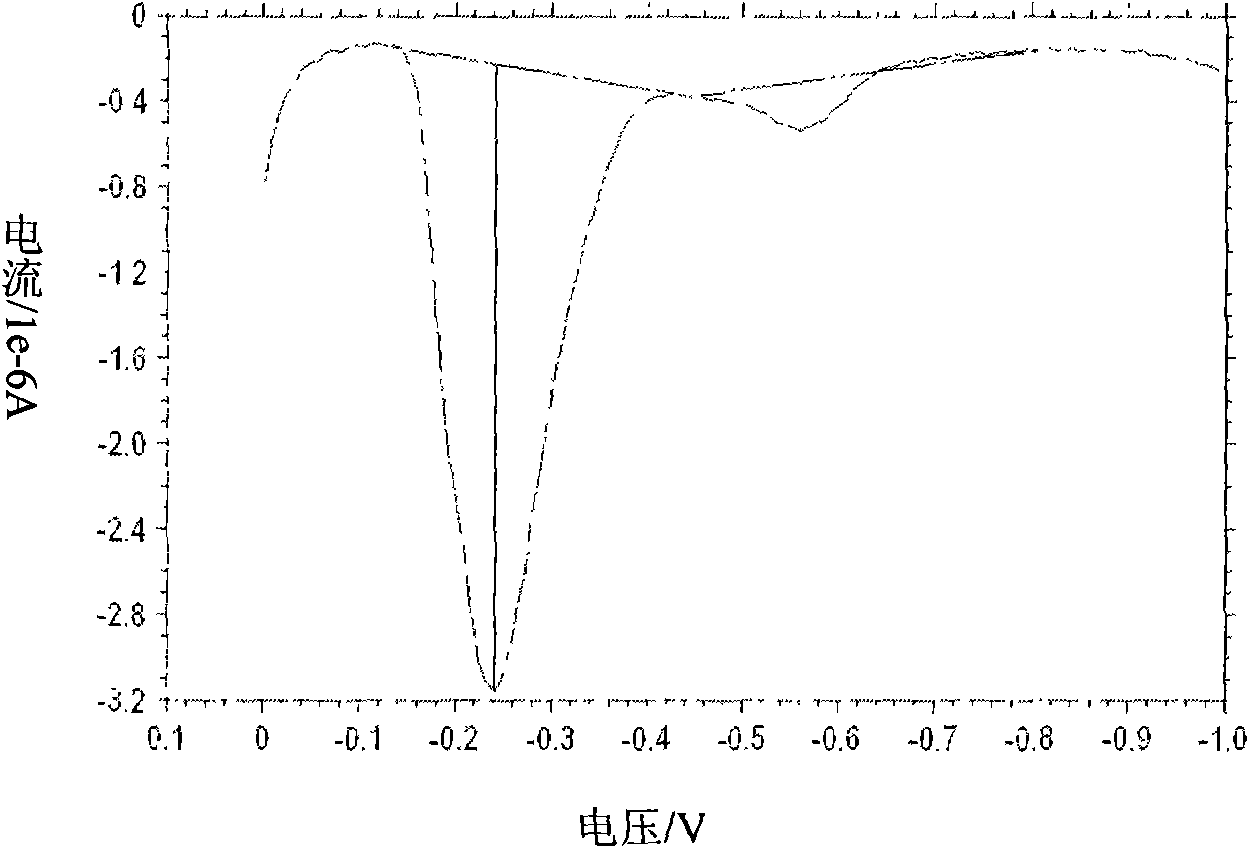
[0030] Embodiment 1: detect the lead ion in the water sample
[0031] Using the above-mentioned standard addition method, prepare a water sample that has been determined to contain a trace amount of lead nitrate (2.5 μM) as the solution to be tested. The detection base solution is 0.2M acetate buffer solution (pH=3.4) containing 10 μg / L mercuric nitrate. The water sample is used as the detection object, and the water body polluted by heavy metal ions is actually detected according to the method of the present invention.
[0032] Take an appropriate amount (about 50 μl) of the sample to be tested, mix it with an equal volume of the detection base solution, use a pipette to absorb 50 μl of the mixed solution, and drop it on the surface of the screen-printed electrode for detection (detection parameters: constant potential is -1.2 V; detection time is 100 seconds).
[0033] Correction of detection results: add a standard substance (internal standard ion) of known concentration ...
Embodiment 2
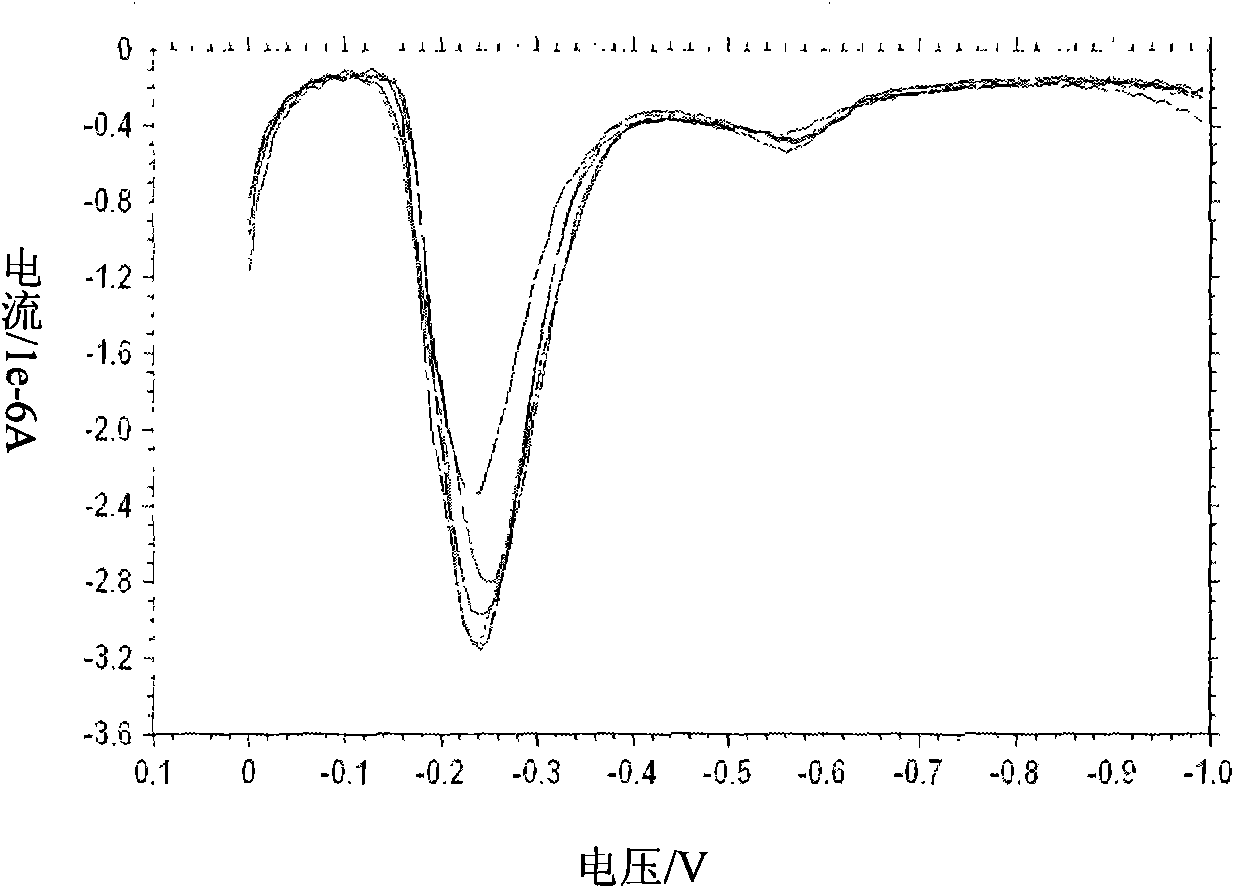
[0036] Embodiment 2: detect the copper ion in the water sample
[0037] Using the detection method described in the present invention, first prepare a water sample determined to contain a small amount of copper acetate (0.5 μM) as the solution to be tested. The detection base solution is 0.2M acetate buffer solution (pH=3.4) containing 10 μg / L mercuric nitrate. This aqueous solution is used as the detection object.
[0038] Take an appropriate amount (about 50 μl) of the sample to be tested, mix it with the same volume of the detection base solution, use a pipette to absorb 50 μl of the resulting mixed solution, and drop it on the surface of the screen-printed electrode for detection (the detection parameter is: a constant potential of -1.0 V; detection time is 100 seconds).
[0039] Correction of detection results: add a standard substance (internal standard ion) of known concentration (Amol / L), the detected concentration is B mol / L, and the detection concentration of the sub...
Embodiment 3
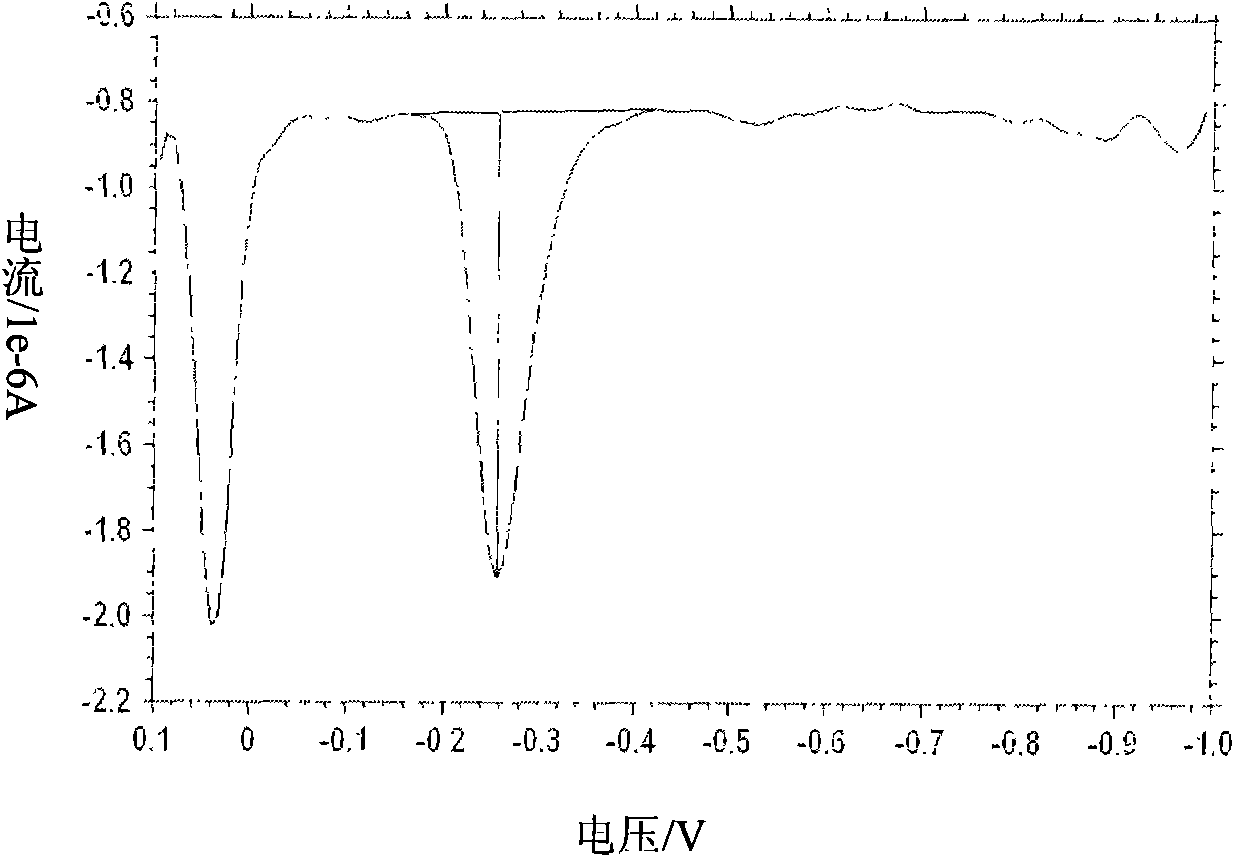
[0041] Embodiment 3: detect the cadmium ion in the water sample
[0042] Using the detection method described in the present invention, a water sample determined to contain a trace amount of cadmium acetate (1 μM) is prepared in advance as the solution to be tested. The detection base solution is 0.2M acetate buffer solution (pH=3.4) containing 10 μg / L mercuric nitrate. Take this water sample as the detection object.
[0043] Take an appropriate amount (about 50 μl) of the sample to be tested, mix it with an equal volume of the detection base solution, use a pipette to absorb 50 μl of the mixed solution, and drop it on the surface of the screen-printed electrode for detection (detection parameters: constant potential is -1.3V; The detection time is 100 seconds).
[0044] Correction of detection results: add a standard substance (internal standard ion) with a known concentration (Amol / L), and the detected concentration is B mol / L; the detection concentration of the substance ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com