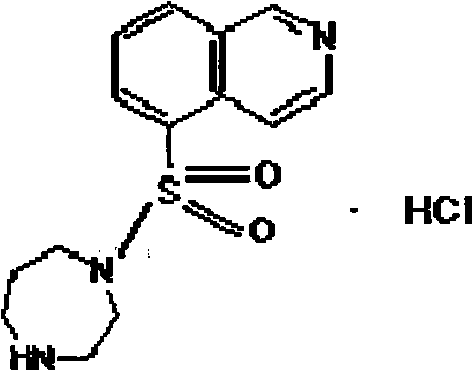
Method for refining sulfonyl isoquinoline derivative
The technology of a sulfonyl isoquinoline and a refining method, which is applied in the field of medicine, can solve the problems of being unsuitable for mass production, long production cycle and high production cost, and achieve the effects of shortening production cycle, low production cost and good product quality.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
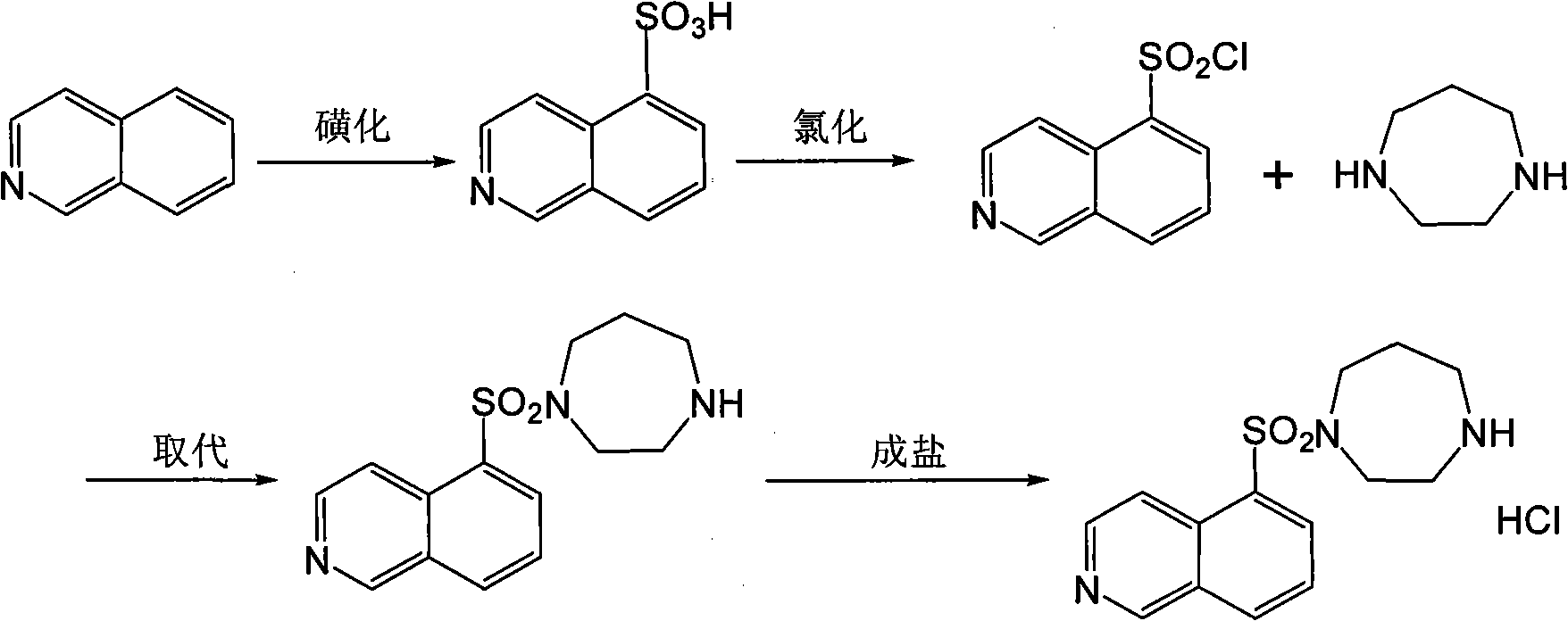
[0025] 1. Substitution reaction:
[0026] After sulfonation and chlorination, 5-sulfonylchloroisoquinoline was obtained in the reaction flask, and 356.0 ml of dichloromethane and 31.4 grams of 1,4-diazepane were added to the reaction flask, and the temperature was lowered to -5 ~0°C, 650 ml of methylene chloride solution containing 29.3 g of 5-sulfonylchloroisoquinoline was added dropwise, and the temperature was always controlled between -5 and 0°C during the dropping process. Then react for 3 hours under the condition of 0-5°C.
[0027] 2. Purification:
[0028] 1) After the substitution reaction is finished, add 150.0 milliliters of purified water to the obtained reaction solution, stir to fully mix the purified water with the above-mentioned solution, let stand to separate the layers, separate the water layer, and use 100.0 milliliters of dichloromethane to back-extract the water layer, Combine the organic layers.
[0029] 2) Below 30°C, the obtained organic layer was c...
Embodiment 2
[0036] 1. Substitution reaction:
[0037] After sulfonation and chlorination, 5-sulfonylchloroisoquinoline was obtained in the reaction flask, and 2.9 liters of dichloromethane and 259.2 grams of 1,4-diazepane were added to the reaction flask, and the temperature was lowered to -5 ~0°C, add dropwise 5.1 liters of dichloromethane solution containing 243.1 g of 5-sulfonylchloroisoquinoline, and keep the temperature between -5 and 0°C throughout the dropping process, and the dropwise addition is completed within 1 to 2 hours. Then react for 3 hours under the condition of 0-5°C.
[0038] 2. Purification:
[0039] 1) After the completion of the substitution reaction, add 1.2 liters of purified water to the obtained reaction solution, stir to fully mix the purified water with the above solution, leave to stand for stratification, separate the water layer, and use 0.9 liters of dichloromethane to back-extract the water layer, The organic layers were then combined.
[0040] 2) Belo...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com