Application of imperatorin in preparing antineoplastic drugs with multi-drug resistance (MDR)
A multi-drug resistance, imperatorin technology, applied in anti-tumor drugs, drug combinations, active ingredients of heterocyclic compounds, etc., can solve problems such as no report on inhibiting proliferation.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] Example 1: Separation and purification of imperatorin from the traditional Chinese medicine Angelica dahurica and identification of its structure
[0021] experimental method
[0022] 1. Separation and purification: the dried roots of Angelica dahurica are ground into powder in a grinder, and weighed to obtain 3 kg of dried Angelica dahurica powder. Soak the powder in petroleum ether and ethyl acetate solvent for 48h, wherein the volume ratio of petroleum ether and ethyl acetate is 2:1. Then pour it into a round-bottomed flask, heat and reflux at about 100°C in a jacketed thermostat for about 2 hours, connect a condenser tube to the top of the round-bottomed flask and continue to pass water to achieve the purpose of condensation and reflux. After cooling, pour out the liquid and filter it, and leave the filter residue in the round bottom flask, and then add an appropriate amount of petroleum ether and ethyl acetate solvent, continue to heat and reflux the Angelica dahu...
Embodiment 2
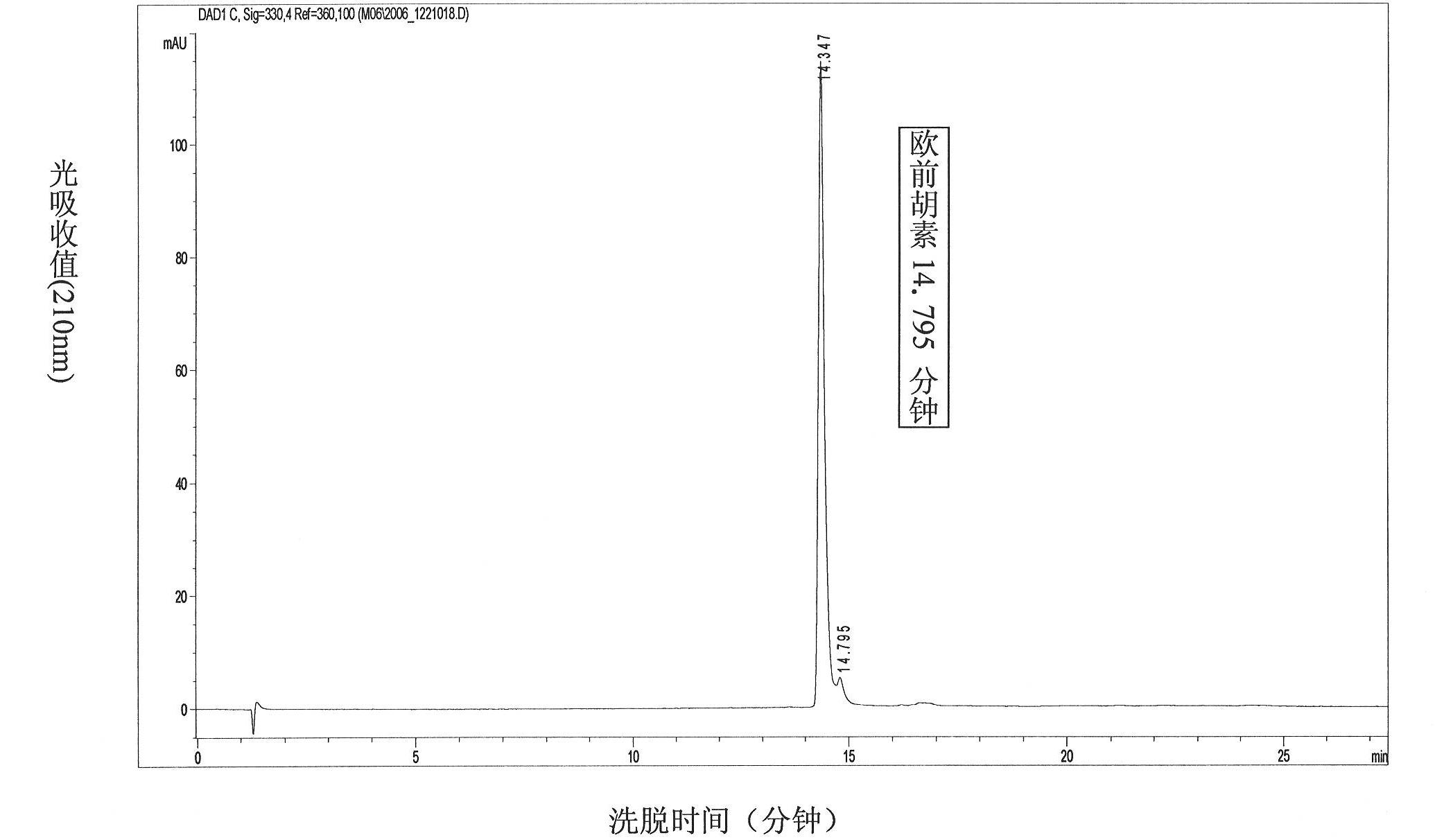
[0025] 2. Structural identification of imperatorin: white cotton-like substance, 1H NMR (500Hz, DMSO) main chemical shift: 1H-NMR: δppm: 7.764 (1H, d, J=9.6Hz, C4-H), 7.691 (1H , d, J=2.0Hz, C12-H), 7.359 (1H, s, C5-H), 6.814 (1H, d, J=2.0Hz, C11-H), 6.370 (1H, d, J=9.6Hz , C3-H), 5.613 (1H, m, C2′-H), 5.007 (2H, d, J=7.2Hz, C1′-H), 1.744, 1.722 (3H each, s, 2×CH3), according to These data are compared with the literature, and it is determined that the monomer compound is imperatorin (Imperatorin), and the molecular formula is C16H1404. figure 1 It is a liquid chromatographic analysis of imperatorin extracted from the traditional Chinese medicine Angelica dahurica. Example 2 Comparison of the Sensitivity of Liver Cancer HepG2 Cells and R-HepG2 to Clinical Antineoplastic Drugs and the Expression of P-glycoprotein
[0026] Experimental materials and methods:
[0027] Liver cancer cell HepG2 was purchased from the American Type Culture Collection in the United States. The drug...
Embodiment 3
[0037] Example 3 Inhibition of imperatorin on proliferation of HepG2 and R-HepG2 in vitro
[0038] Experimental materials and methods: cell source and MTT method are the same as in Example 2
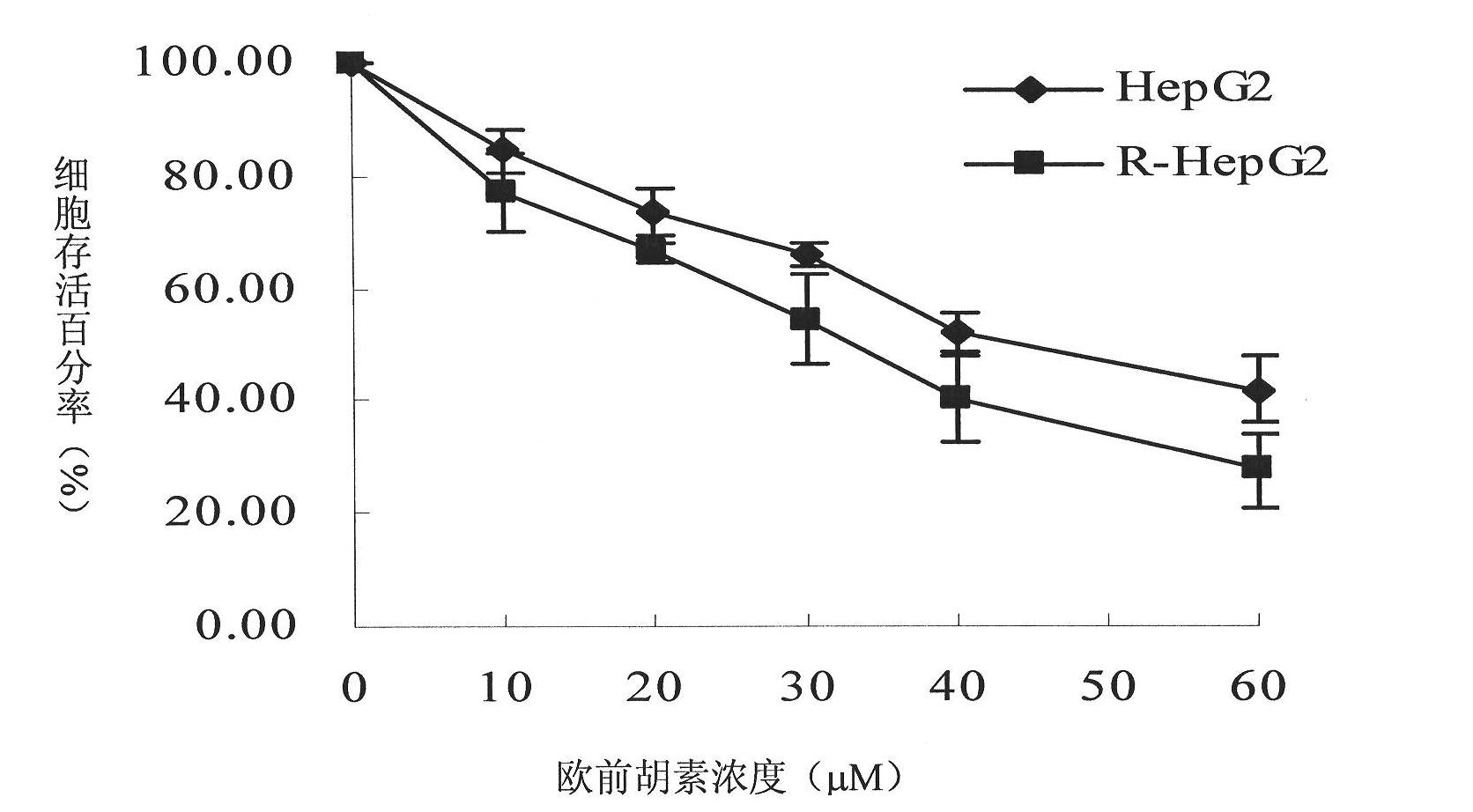
[0039] The experimental results are attached image 3 .
[0040] The inhibition of imperatorin on the proliferation of HepG2 cells and R-HepG2 cells in vitro was dose-dependent, and R-HepG2 cells were more sensitive to it. The half-inhibitory concentration of imperatorin on HepG2 and R-HepG2 after 48 hours of action IC 50 They were 43.3 μM and 28.1 μM respectively, and the drug resistance index RI was 0.65, which was much smaller than that of adriamycin 50 (Table 1), indicating that R-HepG2 had no resistance to imperatorin.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com