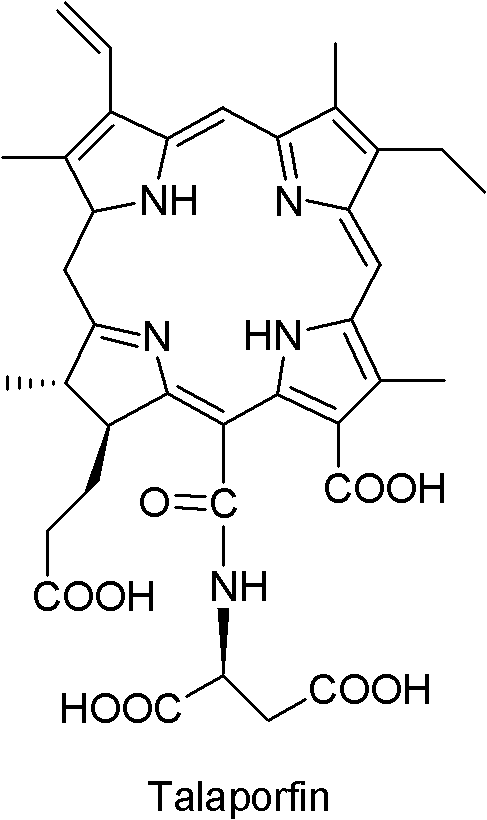
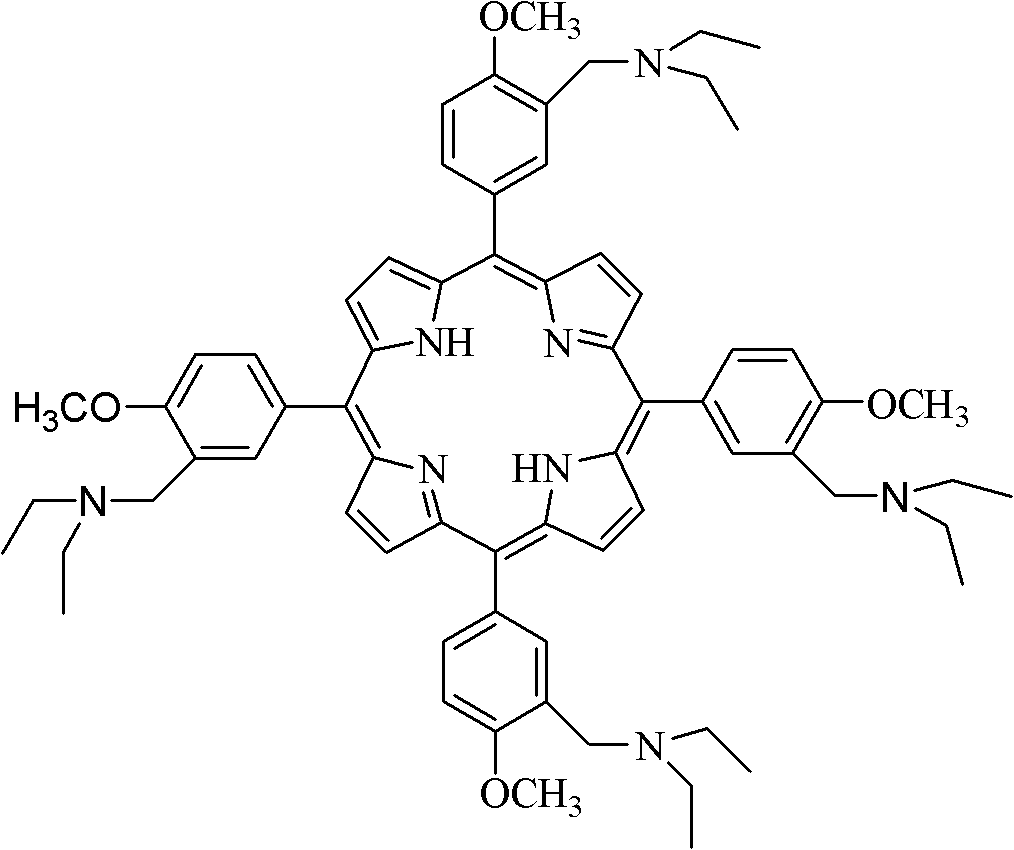
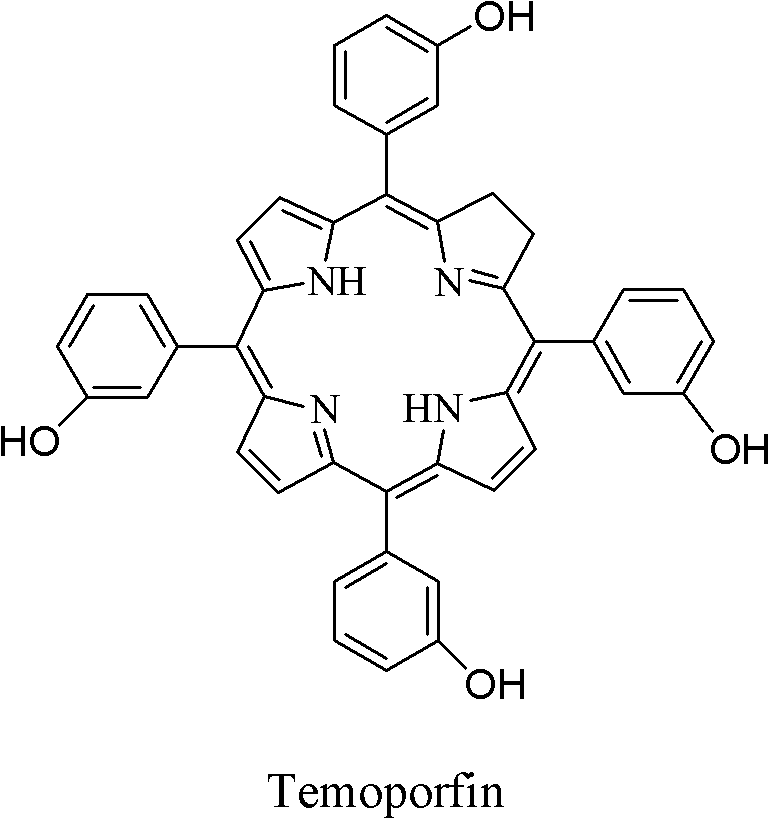
Dihydroporphin photosensitizer as well as preparation method and application thereof
A technology of photosensitizer and chlorin, which is applied in the field of chlorin photosensitizer and its preparation and application, can solve the problems of affecting the effect of photodynamic therapy, less distribution, easy to be oxidized and damaged, and achieve significant killing effect, Enhanced stability and simple preparation method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] Meso-tetra[3-(N,N-diethylaminomethyl)-4-methoxyphenyl]chlorin (meso-tetra[3-(N,N-diethylaminomethyl)-4-methoxyphenyl] Chlorine) synthesis:
[0029] Add 1184g (1.047mmol) meso-tetrakis[3-(N,N-diethylaminomethyl)-4-methoxyphenyl]porphine to a 250mL three-necked flask, add 0.318g (1.696mmol) p-toluenesulfonate hydrazide, and added 0.977g (7.080mmol) of anhydrous potassium carbonate. Evacuate and fill with nitrogen. Finally, add 48mL of pyridine to the three-necked flask, stir evenly, keep heating and reflux in the oil bath under the protection of nitrogen, and add 0.318g (1.696mmol) p-toluenesulfonylhydrazide in the 2nd, 4th, 6th, and 8th hours of the reaction , TLC detection reaction. After the reaction was over, the heating was stopped and allowed to cool naturally. Then it was stirred overnight at room temperature (25°C). Afterwards, 90 mL of ethyl acetate and 40 mL of water were added to the reaction solution, and the mixture was kept at 100° C. in an oil bath for...
Embodiment 2
[0034] Meso-tetra[3-(N,N-diethylaminomethyl)-4-methoxyphenyl]chlorin (meso-tetra[3-(N,N-diethylaminomethyl)-4-methoxyphenyl] Chlorine) synthesis:
[0035]Add 1193g (1.055mmol) meso-tetrakis[3-(N,N-diethylaminomethyl)-4-methoxyphenyl]porphine to a 250mL three-necked flask, add 0.087g (1.72mmol) hydrazine hydrate, And add 0.994g (7.200mmol) of anhydrous potassium carbonate. Evacuate and fill with nitrogen. Finally, 48 mL of pyridine was added to the three-necked flask, stirred evenly, under the protection of nitrogen, kept under heating and reflux in an oil bath, and 0.087 g (1.72 mmol) of hydrazine hydrate was added during the 2nd, 4th, 6th, and 8th hours of the reaction, and detected by TLC reaction. After the reaction was over, the heating was stopped and allowed to cool naturally. It was then stirred overnight at room temperature (25°C). Afterwards, 110 mL of ethyl acetate and 60 mL of water were added to the reaction liquid, and the mixture was kept at 100° C. in an oi...
Embodiment 3
[0037] Photodynamic Antiproliferation Experiment of Photosensitizers on Colon Cancer SW480 Cells
[0038] Tested cells: colon cancer cells SW480
[0039] Test drug: photosensitizer, hematoporphyrin derivative HpD (produced by Beijing Institute of Pharmaceutical Industry).
[0040] Light source: MTZ-1 pulse laser cancer treatment machine; SD2490 laser power measuring instrument.
[0041] Photodynamic anti-tumor cell proliferation experiment: cells in the logarithmic growth phase were digested with trypsin, and the complete medium was resuspended into a cell suspension, which was then inoculated in a 96-well plate, 100 μl per well, placed at 37 ℃5%CO 2 Culture in an incubator, add two different photosensitizers at the same concentration after 24 hours; replace with fresh medium after 48 hours, and then light (XD-635AB photodynamic PDT laser therapy instrument, power 15mW / cm 2 , wavelength 630mm, radiation cells 20min, light dose 18J / cm 2 ); MTT detection was carried out at 7...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com