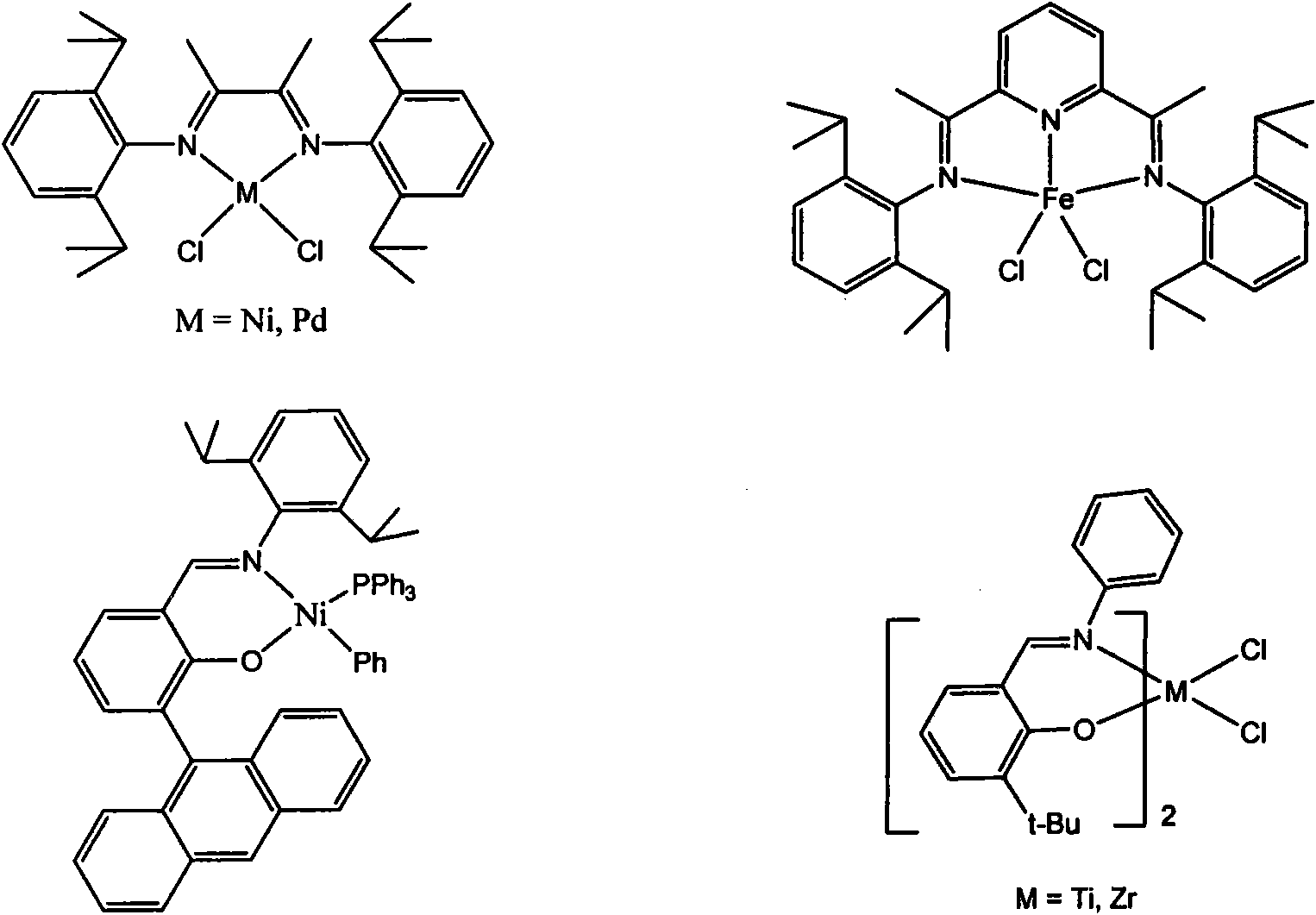
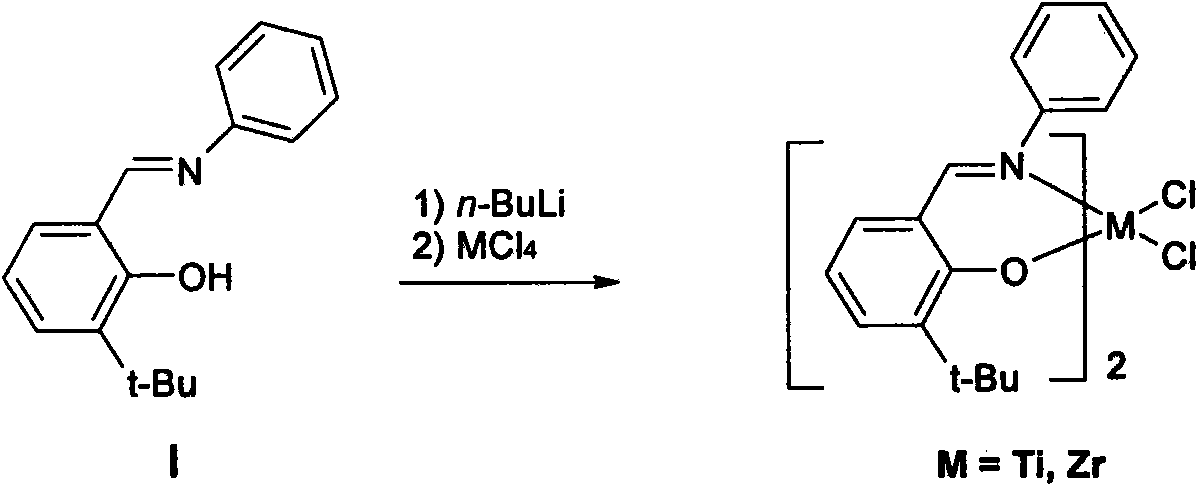
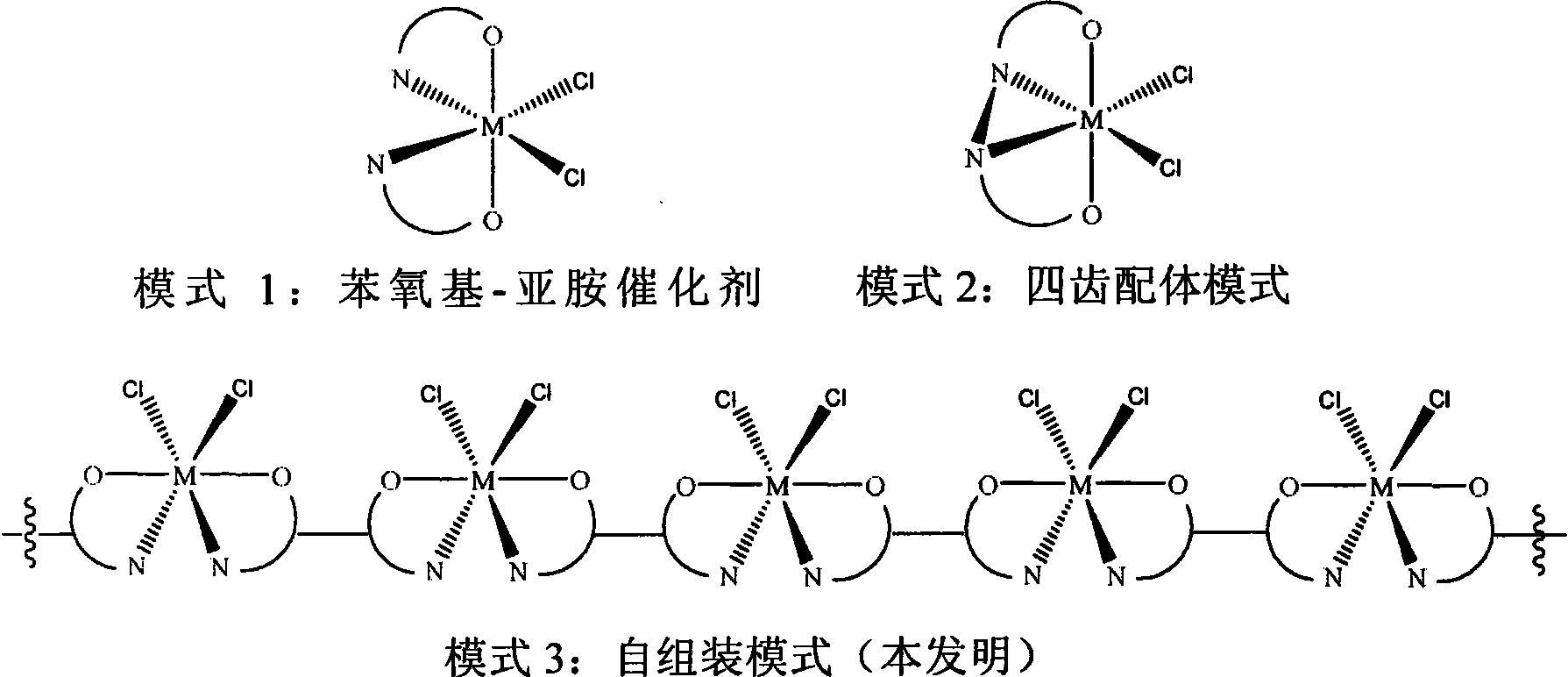
Self-assembled olefin polymerization catalyst
An olefin polymerization and catalyst technology, applied in the field of polyolefins, can solve the problems of low total yield and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0208] Example 1: Preparation of bis-phenoxy-imine ligand (XVIII)
[0209]In a 150 mL dry flask, 4,4'-diaminodiphenylmethane (1.34 g, 6.76 mmol) was dissolved in 25 mL of dry methanol. After stirring for a few minutes, 3-tert-butyl-2-hydroxy-benzaldehyde (2.65 g, 14.87 mmol) was added, followed by a few drops of formic acid. The resulting mixture was stirred at room temperature for 1 hour, then refluxed under argon atmosphere for one day. After cooling to room temperature, the product was isolated by filtration, washed with 12 mL of methanol, and dried in vacuo to obtain 3.45 g of the product as a yellow powder, yield 98%. 1 H-NMR (CDCl 3 , 400MHz, δ): 1.50(s, 18H, -C(CH 3 ) 3 ), 4.04 (s, 2H, -CH 2 -), 6.87-7.42 (multiplet, 14H, aromatic-H), 8.63 (s, 2H, -CH=N-), 13.96 (s, 2H, -OH). 13 C-NMR (CDCl 3 , 400MHz, δ): 29.38, 34.93, 41.04, 118.34, 119.13, 121.37, 129.89, 130.30, 130.62, 137.67, 139.64, 146.66, 160.55, 162.90. Elemental Analysis C 35 h 38 N 2 o 2 (518.71)...
Embodiment 2
[0210] Example 2: Preparation of bis-phenoxy-imine ligand (XIX)
[0211] Bis-phenoxy-imine ligand (XIX) was synthesized using the same method as that of ligand (XVIII), using benzidine (1.06 g, 5.74 mmol) and 3-tert-butyl in 30 mL of anhydrous methanol - 2-Hydroxy-benzaldehyde (2.09 g, 11.49 mmol). 2.80 g of yellow powder was obtained with a yield of 99%. 1 H-NMR (CDCl 3 , 400MHz, δ): 1.51(s, 18H, -C(CH 3 ) 3 ), 6.90-7.71 (multiplet, 14H, aromatic-H), 8.71 (s, 2H, -CH=N-), 13.96 (s, 2H, -OH). 13 C-NMR (CDCl 3 , 400MHz, δ): 29.36, 34.94, 118.41, 119.12, 121.74, 127.87, 130.48, 130.71, 137.72, 138.80, 147.64, 160.61, 163.11. Elemental Analysis C 34 h 36 N 2 o 2 (504.68): Calculated: C 80.92%, H 7.19%, N 5.55%; Found: C 80.98%, H 7.12%, N 5.62%. HRMS (EI, m / z): calculated value 504.2777; measured value: 504.2823 (M + ). Single crystals crystallized from toluene. The X-ray molecular structure is shown in Figure 16 middle. The crystal is monoclinic, space group (sp...
Embodiment 3
[0212] Embodiment 3: Preparation of phenoxy-imine ligand (I)
[0213] In a 100 mL dry flask, aniline (1.44 g, 15.46 mmol) was dissolved in 25 mL of dry methanol with stirring. 3-tert-butyl-2-hydroxy-benzaldehyde (2.5 g, 14.03 mmol) was then added followed by a few drops of formic acid. The resulting mixture was stirred at room temperature for 1 hour, then refluxed under argon atmosphere for 8 hours. After cooling to room temperature, the methanol was removed under vacuum to give a yellow residue, which was purified by column chromatography eluting with hexane / ethyl acetate (10:1) to give 3.2 g of the product as a light yellow oil, Yield 90%. 1 H-NMR (CDCl 3 , 400MHz, δ): 1.54 (s, 9H, tert-butyl), 6.91-7.48 (m, 8H, aromatic-H), 8.66 (s, 1H, -CH=N-), 13.97 (s, 1H, -OH). 13 C-NMR (CDCl 3 , 400MHz, δ): 29.39, 34.96, 118.37, 119.10, 121.23, 126.75, 129.41, 130.39, 130.71, 137.69, 148.51, 160.58, 163.42.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com