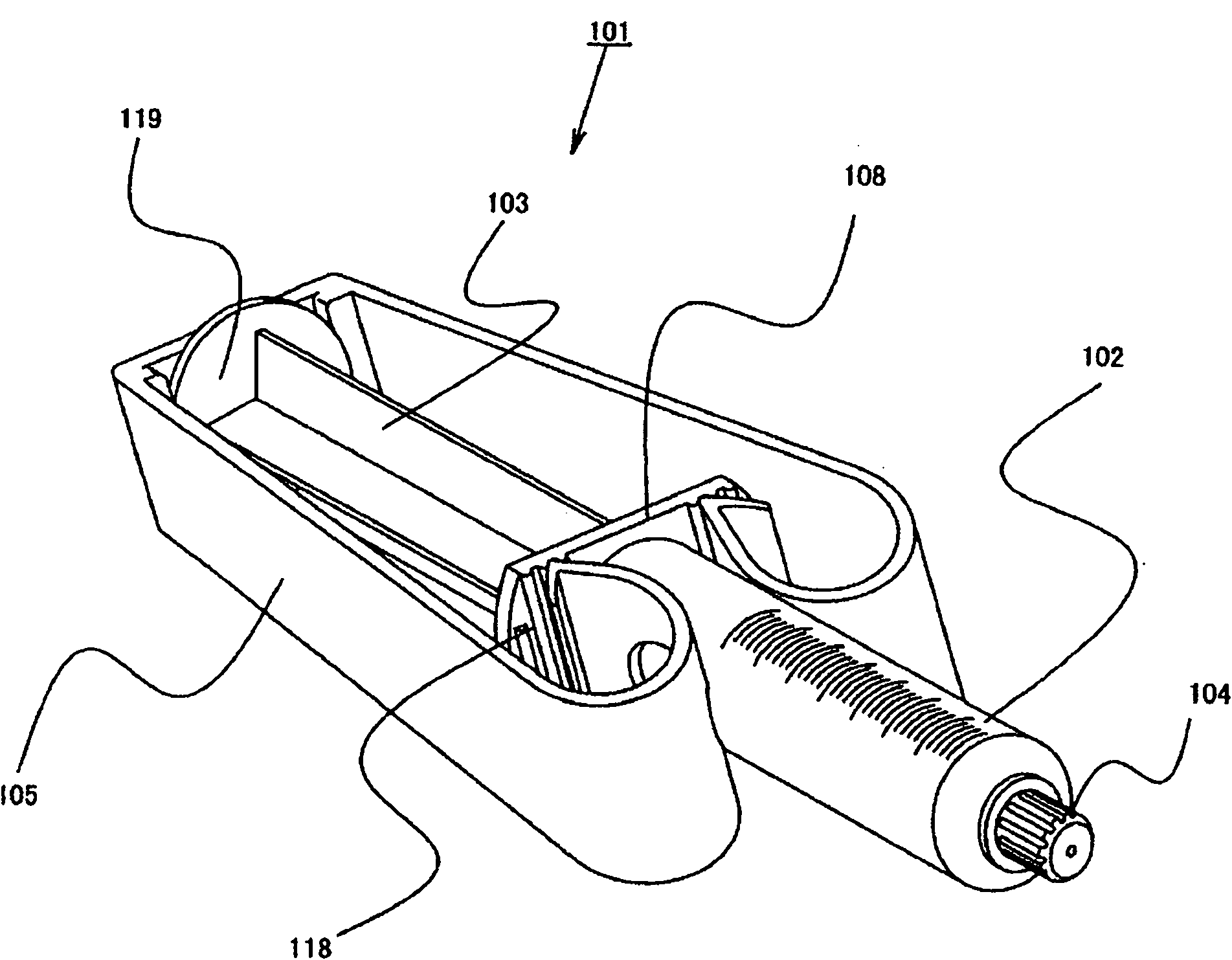
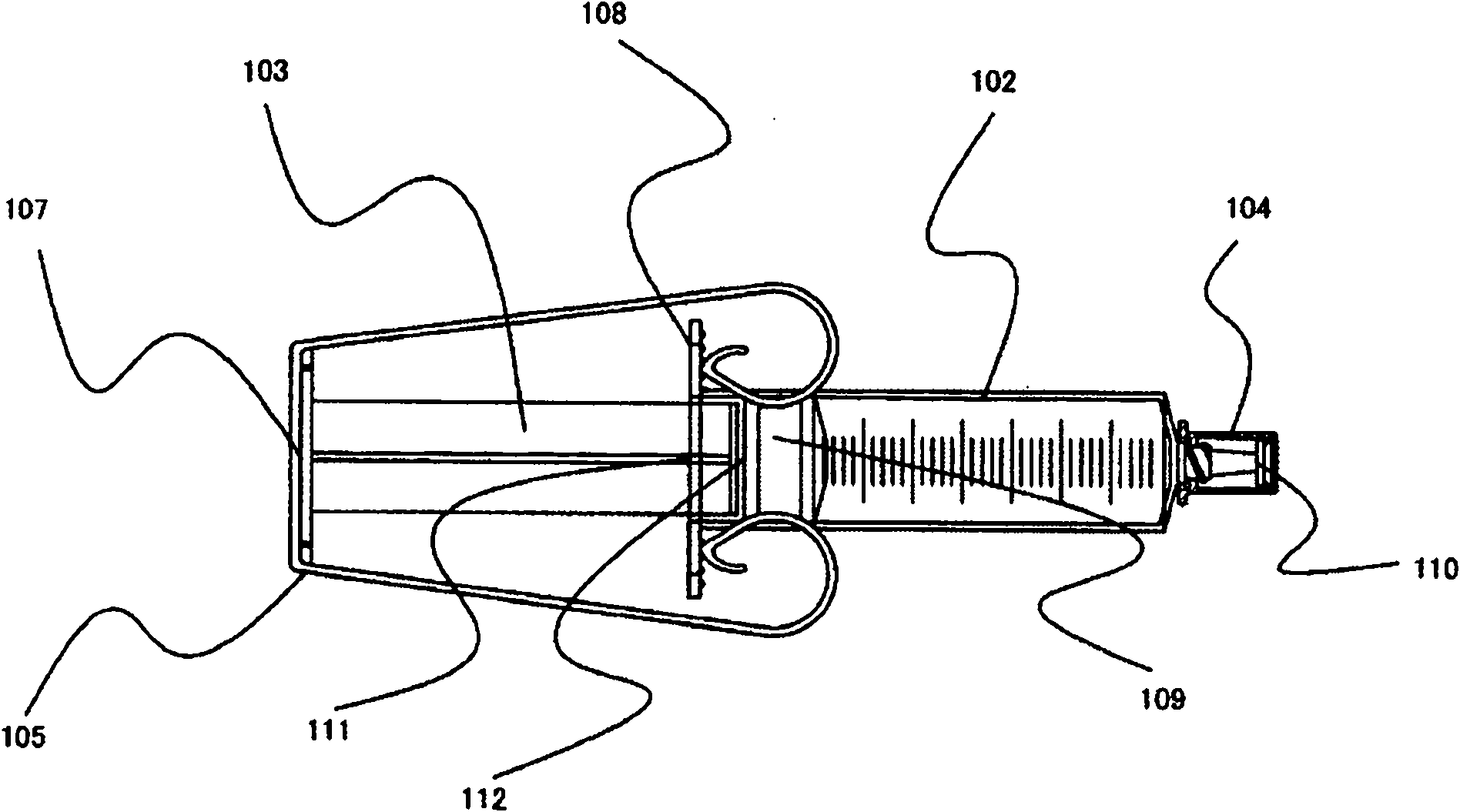
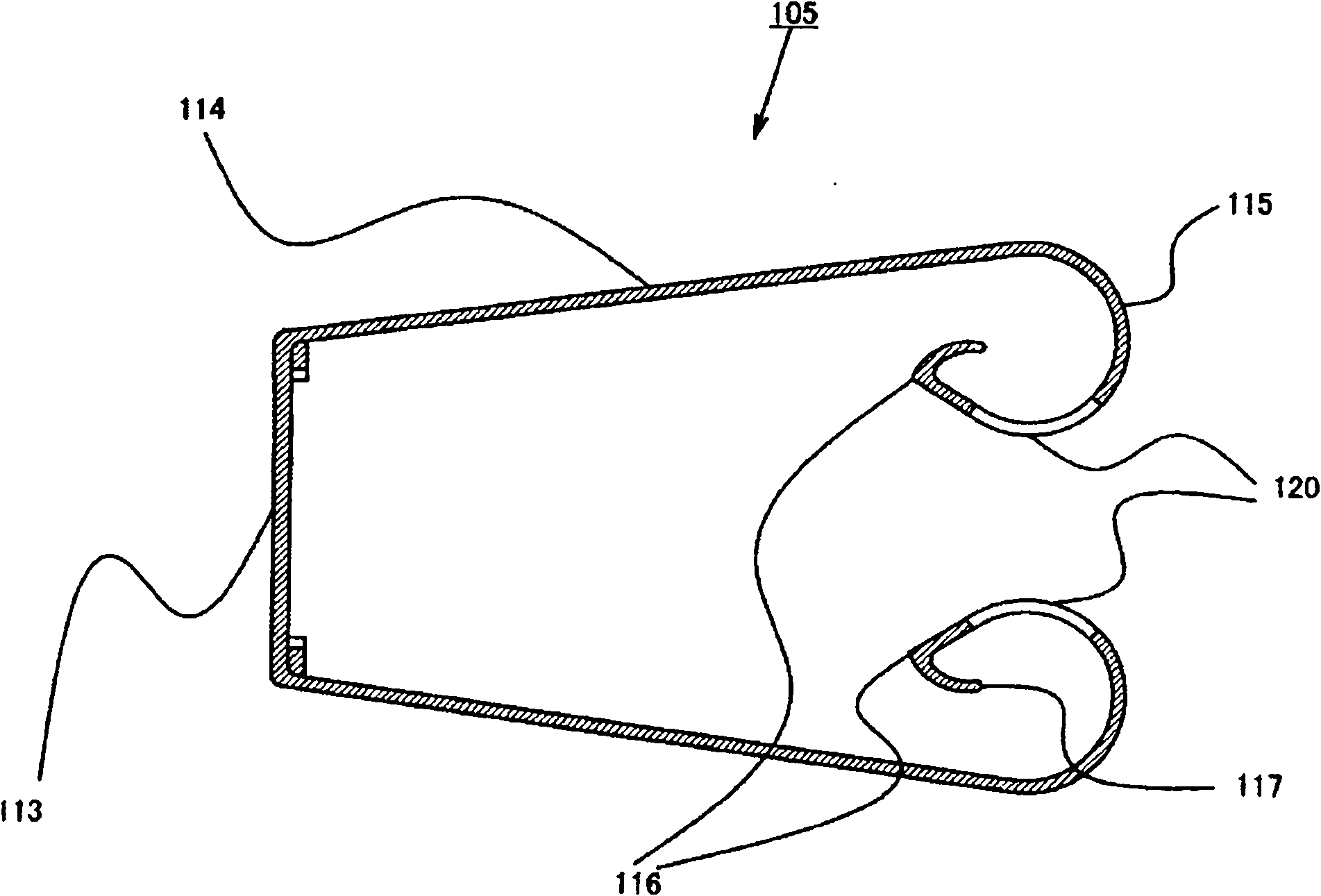
Drug container
A technology for medicines and containers, which is applied to the field of containers for liquid medicines, can solve the problems of not being able to completely prevent air bubbles, promoting protein deterioration, and insufficient injection volume.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
experiment example 1
[0080] exist Figure 10 The measurement device used in this experimental example is shown in .
[0081] In a commercially available polypropylene syringe 501 (TERUMO SYRINGE, manufactured by Terumo Co., Ltd.) of empty 10 ml (indicating that it is sold on the market as a capacity of 10 ml, the same below), fill it with 4 so that there is no gas part. The stored water was cooled at °C, and a pressure gauge 502 (PG-35 manufactured by Nidec Copal Electronics Co., Ltd.) was connected through a luer connector 505 .
[0082] The packing of this commercially available syringe is a soft polyolefin-based elastomer mixed with a styrene-based elastomer.
[0083] The push piece fixing pin 504 is used to fix the push piece so that its relative position with the outer cylinder does not change, preventing the pressure applied to the water from decreasing due to the movement of the gasket. Brass or stainless steel is used for materials of auxiliary parts other than the syringe such as the pr...
experiment example 2
[0089] After moving to an oven at 50°C for 1 hour, adjust the pressure to the set value, and then use the same syringe under the same measurement conditions as in Experimental Example 1, except for long-term storage while correcting the set pressure every day. experimented. The results are shown in Table 2.
[0090] (Table 2) Bubble change with time at 50°C
[0091]
[0092] According to the results of Experimental Example 2, by continuously applying a pressure of 20 kPa or more to the aqueous liquid in the syringe, the air bubbles in the syringe disappeared, and the disappearance speed became faster as the pressure increased, and it was confirmed that the bubbles disappeared in about 2 days.
[0093] Also, even assuming that the set pressure is set to 20 kPa, the pressure decreases over time. used in this experiment example Figure 10 In the measurement device, since the volume inside the syringe is basically constant, the generation or disappearance of a very small amo...
experiment example 3
[0103] Change the storage temperature to room temperature, except to use commercially available syringes of various volumes (5ml: VITAJECT manufactured by Terumo Corporation; 10ml: TERUMO SYRINGE manufactured by Terumo Corporation; 20ml: KC1MEDIJECT manufactured by Terumo Corporation) In addition, by the same method as in Experimental Example 2, it was confirmed that the air bubbles disappeared when stored under pressure at 25 kPa.
[0104] Among the constituent members of each syringe, the outer cylinder was made of polypropylene, and the packing was made of olefin-based elastomer including styrene-based elastomer.
[0105] The results are shown in Table 3.
[0106] (Table 3) Disappearance of air bubbles in polypropylene syringes of various capacities
[0107]
[0108]
[0109] From the results of Experimental Example 3, it was confirmed that air bubbles were present in all the syringes when the temperature was raised from the refrigerated storage state at 4° C. to roo...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com