Process for continuously producing isobutyric acid
A technology of isobutyric acid, continuous method, applied in the separation/purification of carboxylic acid compounds, preparation of carboxylate, chemical instruments and methods, etc., can solve problems such as difficulty in realizing continuous production
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
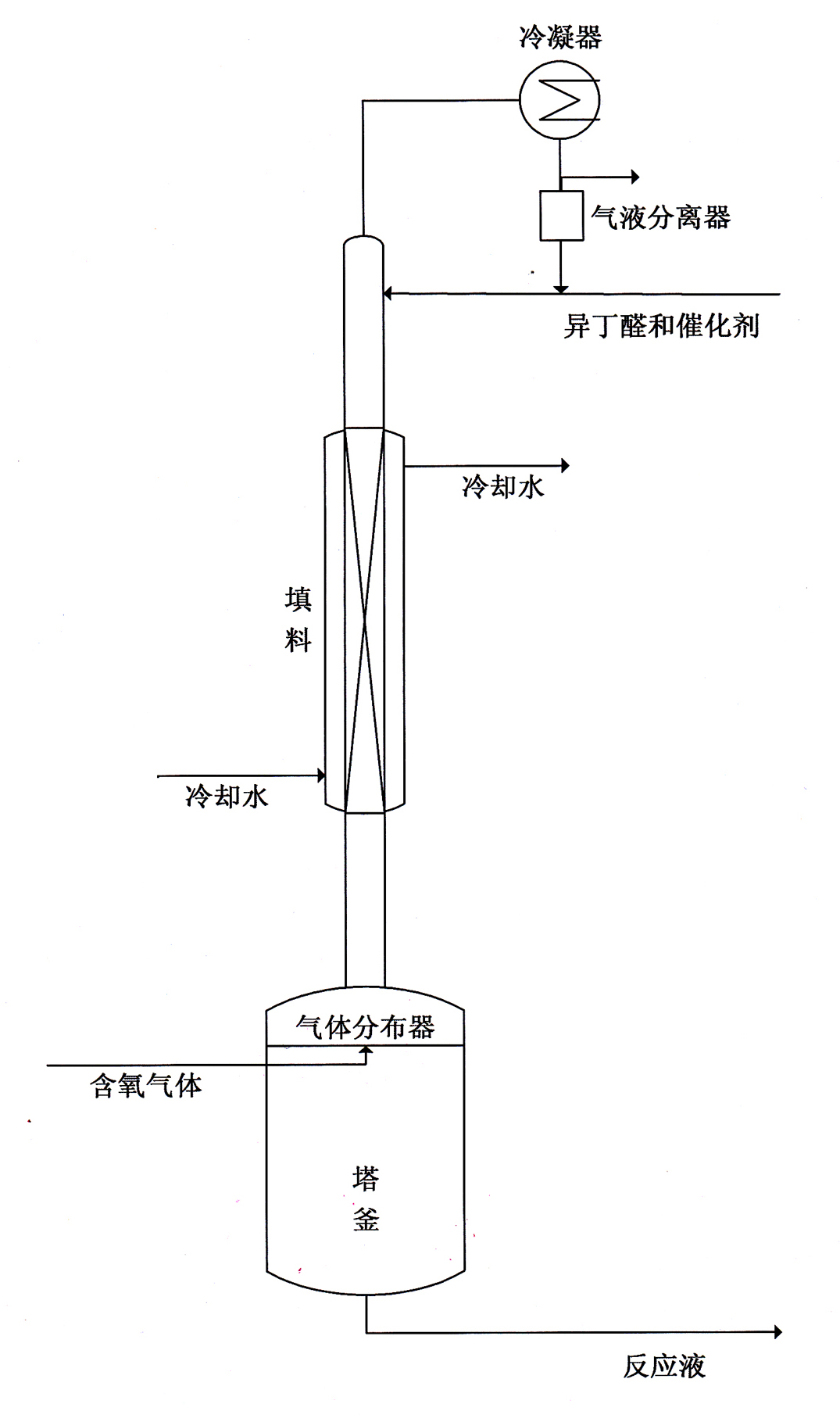
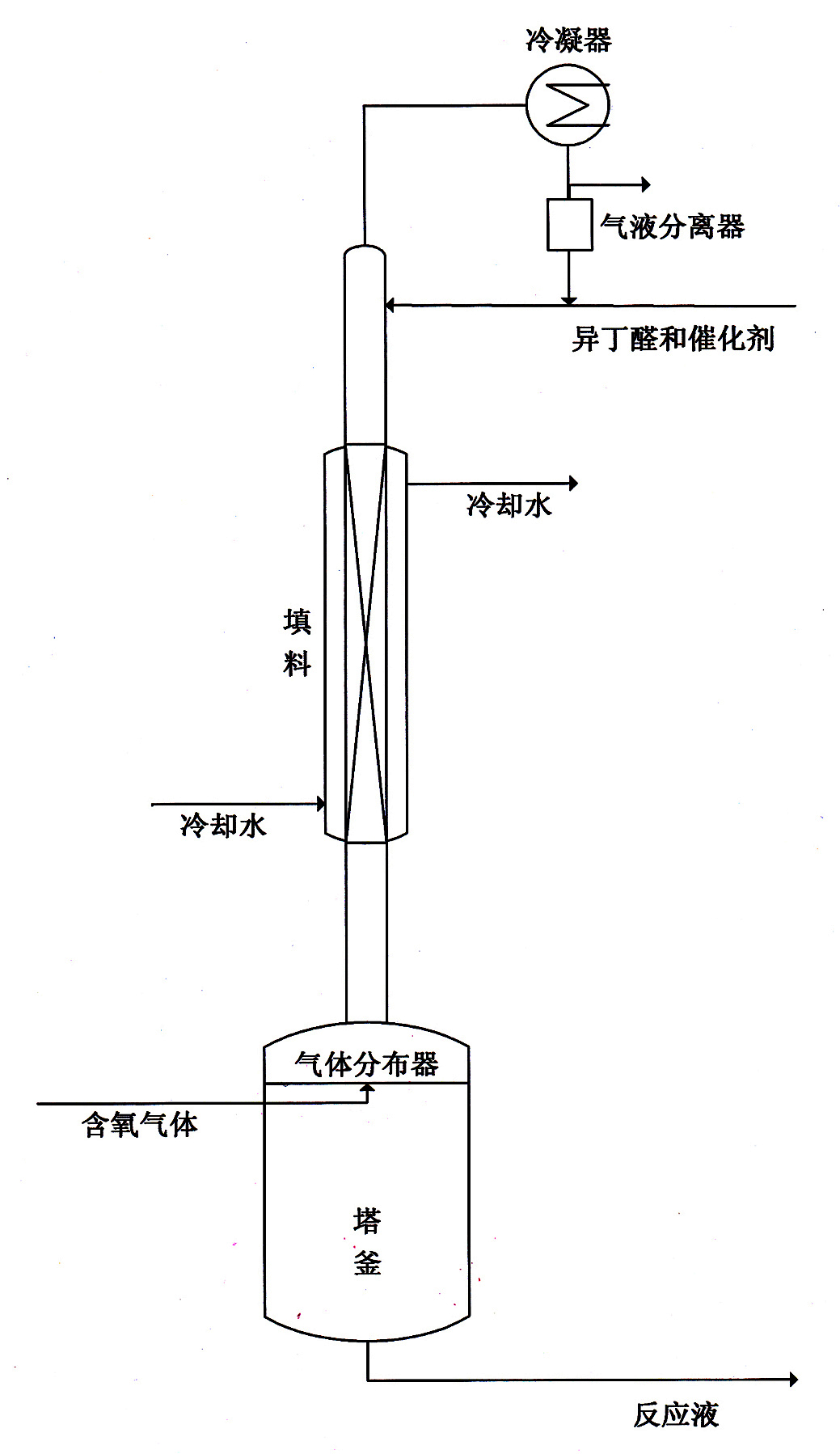
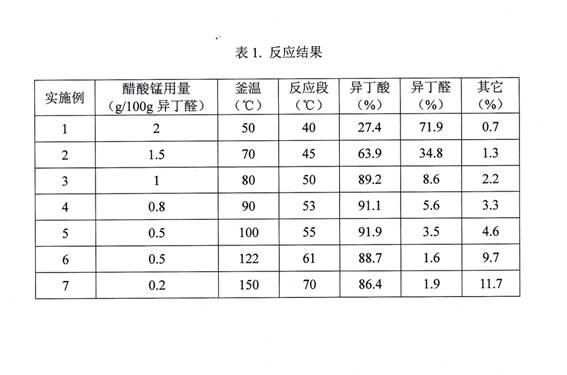
[0013] Embodiment 1, the reactive distillation device is made of reaction kettle, rectification tower body and the condenser and gas-liquid separator at the atmospheric pressure outlet of tower top, reaction kettle 2L, rectification tower internal diameter 8cm, height 2m, in the middle of tower body It is equipped with a packing with a height of 1.8m (the packing can be porcelain ring or stainless steel packing). The reaction material is isobutyraldehyde, the reaction section of the rectification tower is equipped with a jacketed water cooling device, and the catalyst is an isobutyric acid solution with a weight content of 10% manganese acetate. The isobutyraldehyde feed rate is 40g / h, the configured catalyst feed rate is 8g / h, the air flow rate is 50L / h, and the reaction product is continuously discharged to keep the liquid level in the reactor at the bottom of the tower constant (the same below). Its discharge rate is 50.5g / h, and the gas flowing out from the top of the towe...
Embodiment 2
[0014] Embodiment 2, reaction unit, feeding and discharging mode and product analysis method are with embodiment 1, and catalyzer is the manganese acetate isobutyric acid solution that weight content is 15%, and isobutyraldehyde feed rate is 40g / h, and catalyzer feed rate is 4g / h, the discharge rate of the reaction product is 50g / h, the air flow rate is 50L / h, the kettle temperature is 70°C, and the reaction zone temperature is 45°C. The reaction results are shown in Table 1.
Embodiment 3
[0015] Embodiment 3, reaction unit, feeding and discharging mode and product analysis method are with embodiment 1, and catalyzer is the manganese acetate isobutyric acid solution that weight content is 20%, and isobutyraldehyde feed rate is 40g / h, and catalyzer feed rate is 2g / h, the discharge rate of the reaction product was 50.4g / h, the air flow rate was 50L / h, the kettle temperature was 80°C, and the reaction zone temperature was 50°C. The reaction results are shown in Table 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com