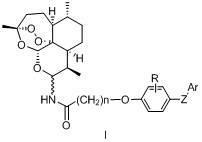
Artemisinin derivatives and application thereof
A derivative, halogen technology, applied in the field of medicine
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0119] Add dihydroartemisinin (200 mmol, 56.8 g) and sodium azide 39 g (600 mmol) into (800 mL) dichloromethane solution, cool to 0-5°C after addition, and add in batches Chlorotrimethylsilane (300 mmol, 38.1 mL), and then add a catalytic amount of sodium iodide, rise to room temperature and react for 28 hours. After the reaction is complete, pour the reaction solution into 800 mL of water, extract with dichloromethane, and combine the extracted liquid, washed with water, and dried over anhydrous sodium sulfate. Evaporated to dryness to obtain oil, separated by column chromatography to obtain optically pure 10 α -Azidodihydroartemisinin 3.0 g and 10 β - Azidodihydroartemisinin 29.0 g, the yields were 5% and 47%, respectively, MS: 332.2 (M+ Na).
[0120] Step B: 10 α- Preparation of Aminodihydroartemisinin
[0121] will be 10 α - Azidodihydroartemisinin 3.0 g (9.7 mmol) was gradually added to the tetrahydrofuran solution, and at room temperature, 5.1 g (19.4 mmol) of triph...
Embodiment 2
[0132] Example 3:
[0133] (10 S )- N -{4-[3-(4-Methylsulfonylphenyl)-2-( E )-acryloyl]phenoxyacetyl}aminodihydroartemisinin
Embodiment 3
[0135] Example 4:
[0136] (10 S )- N -{4-[3-(4-Trifluoromethoxyphenyl)-2-( E )-acryloyl]phenoxyacetyl}aminodihydroartemisinin
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com