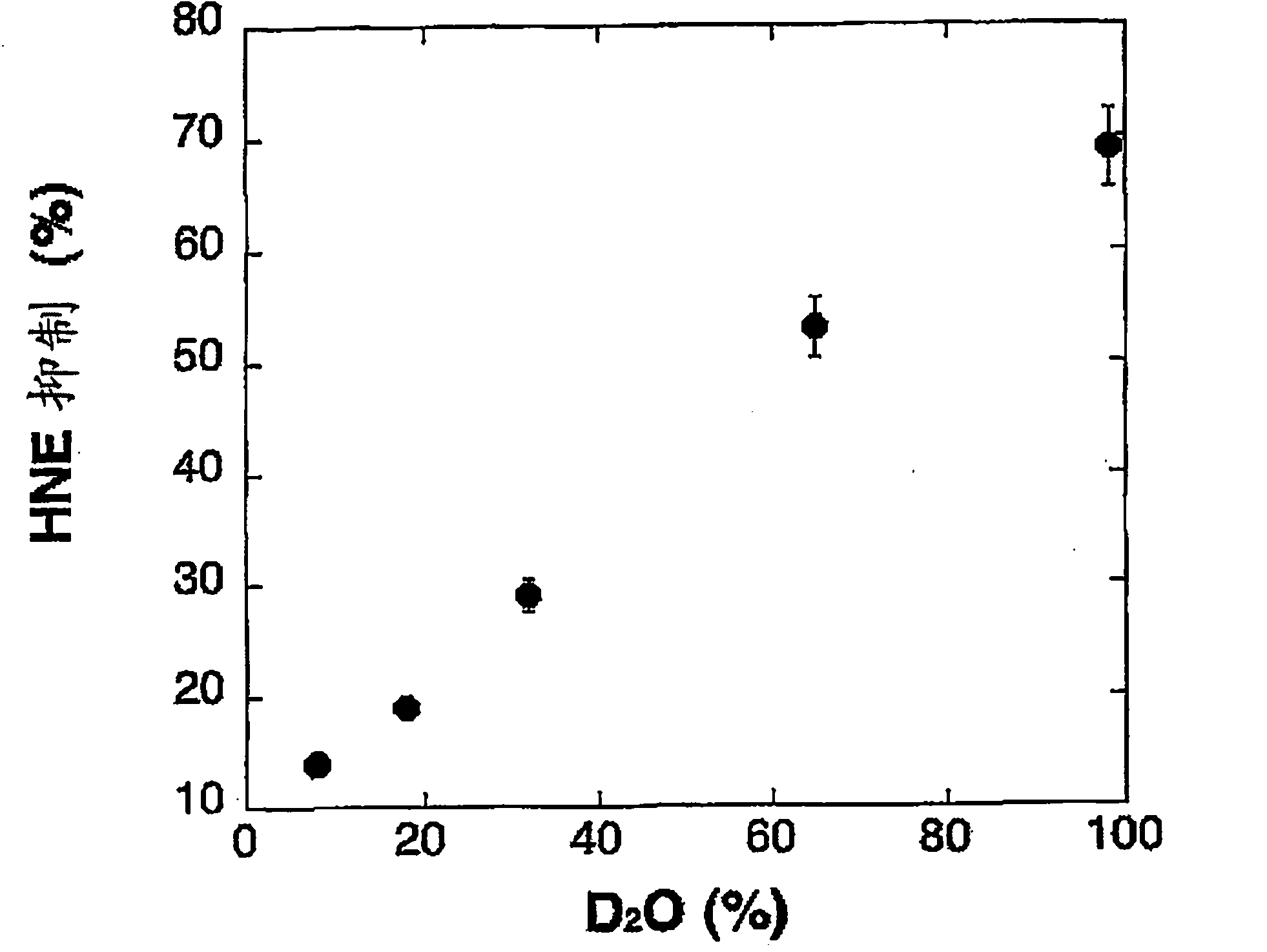
Use of deuterium oxide as an elastase inhibitor
A technology of elastase and deuterium oxide, applied in the field of deuterium oxide, can solve the problems of limited application due to molecular size, low bioavailability, non-specificity, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0167] Example 1: Preparation of acrylic acid-based hydrogels
[0168] 2.0% by weight of Carbopol 980 (manufacturer: Noveon, Inc., 9911 Brecksville Rd., Cleveland, OH 44141-3247, USA) was dissolved in neat D by stirring in divided portions. 2 O, pure H 2 O or by D 2 O and H 2 O in the mixture, then titrated to pH 6.8 by titrating 10 M NaOH solution. The carboxyl groups of the polyacrylic acid are then crosslinked with the basic hydroxyl groups by the addition of NaOH, resulting in a colorless, transparent and optically clear acrylic gel (Carbopol gel) (D 2 O-Carbopol gel, H 2 O-Carbopol gel, D 2 O / H 2 O-Carbopol gel) was stored at room temperature for at least 24 hours until further application.
Embodiment 2
[0169] Embodiment 2: Preparation of hydrogel based on siloxane (Silicon)
[0170] 3.0% by weight of hexamethyldisiloxane (trade name SILMOGEN CARRIER from DOW Corning) and 1% by weight of ethanol were dissolved in neat D 2 O, pure H 2 O or by D 2 O and H 2 in a mixture of O. The above solution was then immediately mixed with the gel prepared according to Example 1 (Carbopol gel) in a weight ratio of 1:2 (silicone solution:Carbopol gel) under vigorous stirring until an optically clear gel was produced. (silicone gel) (D 2 O silicone gel, H 2 O silicone gel, D 2 O / H 2 O silicone gel). Store the gel at room temperature for at least 24 hours until further use.
Embodiment 3
[0171] Example 3: Preparation of alginate-based hydrogels
[0172] 4.0% by weight of sodium alginate (sodium alginate) (manufacturer: GmbH Armstadt, Deutschland) dispersed in pure D in divided portions by stirring 2 O, pure H 2 O or by D 2 O and H 2 O in the mixture, then titrated to pH 7.0 by titrating 10 M NaOH solution. The resulting tan, transparent gel (alginate gel) (D 2 O alginate gel, H 2 O alginate gel, D 2 O / H 2 O alginate gel) was stored at room temperature for at least 24 hours until further application.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com