Modified double resin ion exchanger, manufacturing method and usage thereof
A technology of ion exchangers and ion exchange resins, applied in the field of ion exchangers, can solve the problems of waste sulfuric acid reuse restrictions, control technology, high energy consumption, high expenditure, and inability to exchange ions, etc. The preparation method is simple and easy to operate, The effect of long service life and not easy to break
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
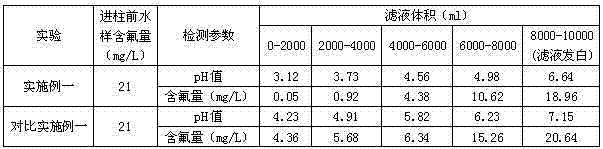
Embodiment 1
[0055] H-type styrene resin is 001×4 strongly acidic styrene hydrogen ion exchange resin produced by Jiangsu Linhai Resin Company; H-type acrylic resin is D113 macroporous weakly acidic acrylic hydrogen ion exchange resin produced by Jiangsu Linhai Resin Company resin.
[0056] Prepare aluminum sulfate solutions with a mass percentage concentration of 8% and 4% by conventional methods.
[0057] Prepare the modified double resin ion exchanger as follows:
[0058] A. Preparation of gel strong acid Al-type ion exchange resin: Fill the exchange column with 50ml of 001×4 strong acidic styrene-based hydrogen ion exchange resin at normal temperature and pressure, and inject 300ml of sulfuric acid with a mass percentage concentration of 8%. Aluminum solution, soaked for 2h, then washed with deionized water to prepare 50.2ml gel strong acid Al-type ion exchange resin, for subsequent use;
[0059] B. Preparation of macroporous weakly acidic Al-type ion exchange resin: Fill 50ml of D11...
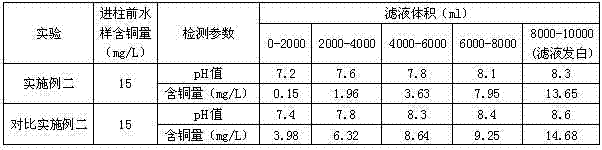
Embodiment 2
[0070] The modified dual-resin ion exchanger prepared in Example 1 was used to carry out the copper removal ion exchange experiment, and the experimental results are shown in Table 2. According to calculation, the copper removal capacity of the modified double resin ion exchanger in this embodiment is 6.21 mg / l when converted to sodium resin.
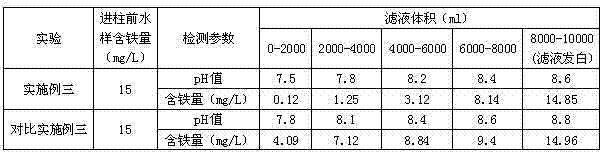
Embodiment 3
[0076] H-type styrene resin is 001X7 strongly acidic styrene-based hydrogen ion exchange resin produced by Jiangsu Sekesaisi Resin Co., Ltd.; H-type acrylic resin is HD-2 macroporous resin produced by Shanghai Huazhen Technology Co., Ltd. Weakly acidic acrylic hydrogen ion exchange resin.
[0077] Prepare aluminum pyrosulfate solutions with mass percentage concentrations of 6% and 3% according to conventional methods.
[0078] Prepare the modified double resin ion exchanger as follows:
[0079] A. Preparation of gel strong acid Al-type ion exchange resin: Fill the exchange column with 50ml of 001X7 strong acid styrene-based hydrogen ion exchange resin at normal temperature and pressure, and inject 300ml of aluminum sulfate solution with a mass percentage concentration of 6%. solution, soaked for 1h, then cleaned with deionized water, and set aside;
[0080] B. Preparation of macroporous weakly acidic Al-type ion exchange resin: Fill 50ml of HD-2 type macroporous weakly acidi...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com