Darifenacin hydrobromide sustained-release tablet and preparation method
A technology of darfenazine hydrobromide and sustained-release tablets, which is applied in the new field of medicine, can solve the problems of poor production controllability, increased material consumption, and small adjustable space, so as to reduce production costs, reduce material consumption, The effect of reducing production costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
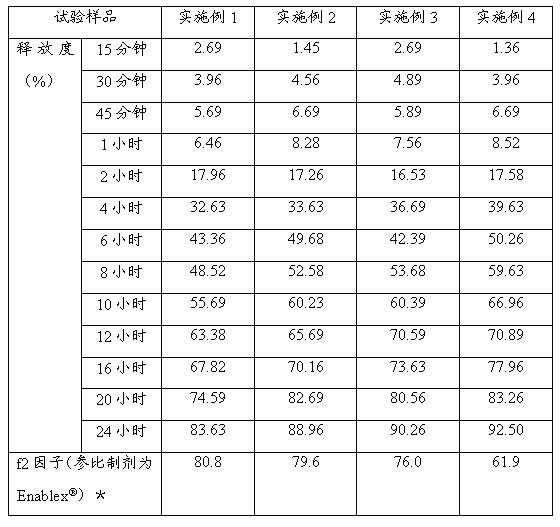
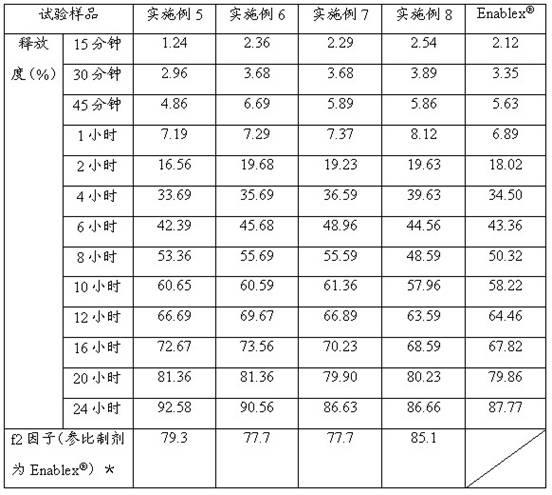
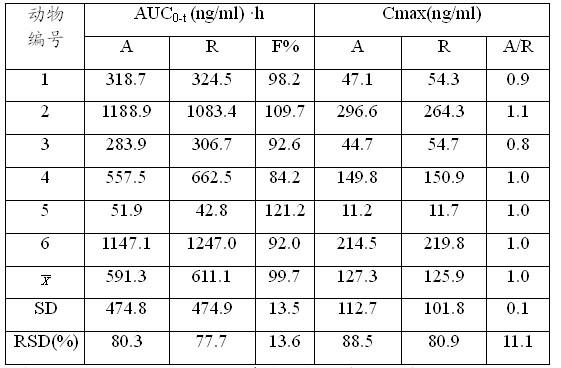
Examples
Embodiment 1
[0024] Carbomer 974P 5.6g
[0025] Calcium hydrogen phosphate 133.47g
[0026] Lactose 50.0g
[0027] Magnesium Stearate 2.0g
[0028] Total weight 200g
[0029] Makes 1000 pieces
[0030] Pass darfenacine hydrobromide, carbomer 974P, calcium hydrogen phosphate and lactose through an 80-mesh sieve, and mix them evenly with a three-dimensional mixer. Lubricant magnesium stearate is added to the above powder, mixed evenly in a three-dimensional mixer, f8mm shallow concave punched tablets, and coated with gastric-soluble coating powder.
[0031] Example 2
Embodiment 2
[0033] Carbomer 971P 2g
[0034] Calcium hydrogen phosphate 137.07g
[0035] Lactose 50.0g
[0036] Magnesium Stearate 2.0g
[0037] Total weight 200g
[0038] Makes 1000 pieces
[0039] Pass darfenacine hydrobromide, carbomer 971P, calcium hydrogen phosphate and lactose through an 80-mesh sieve, and mix them evenly in a three-dimensional mixer. Lubricant magnesium stearate is added to the above powder, mixed evenly in a three-dimensional mixer, f8mm shallow concave punched tablets, and coated with gastric-soluble coating powder.
[0040] Example 3
Embodiment 3
[0042] Carbomer 934P 10g
[0043] Calcium hydrogen phosphate 129.07g
[0044] Lactose 50.0g
[0045] Magnesium Stearate 2.0g
[0046] Total weight 200g
[0047] Makes 1000 pieces
[0048] Pass darfenacine hydrobromide, carbomer 934P, calcium hydrogen phosphate and lactose through an 80-mesh sieve, and mix them evenly in a three-dimensional mixer. Lubricant magnesium stearate is added to the above powder, mixed evenly in a three-dimensional mixer, f8mm shallow concave punched tablets, and coated with gastric-soluble coating powder.
[0049] Example 4
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com