Load type solid catalyst for heterogeneous phase Fenton system and application thereof in water treatment
A solid catalyst, supported technology, applied in the direction of oxidized water/sewage treatment, etc., can solve the problems of decreased treatment efficiency, reduced catalytic performance, and ineffective production of active intermediates.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0061] The aqueous solution used in this example is deionized water in which 100 ppm phenol is dissolved.
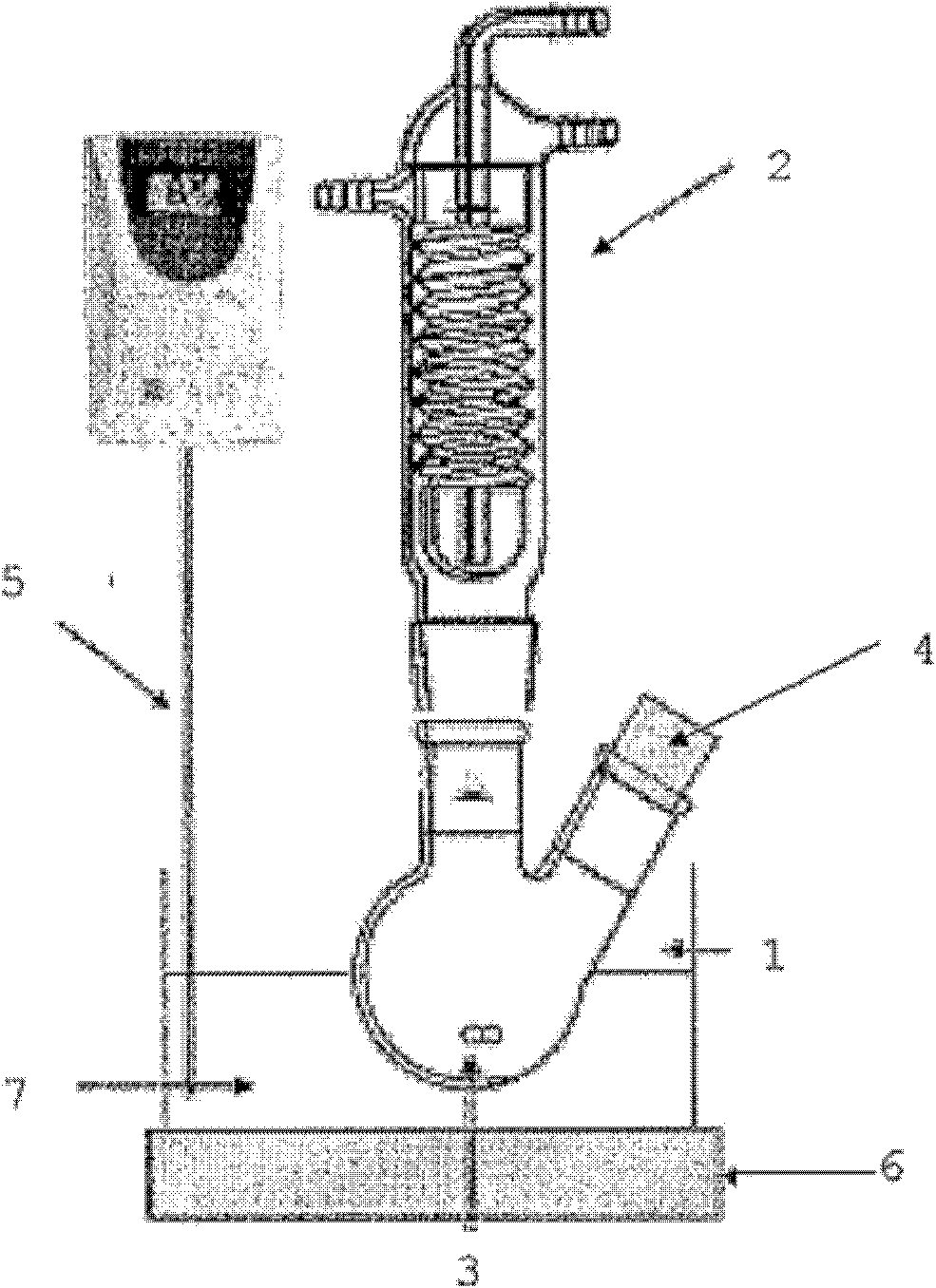
[0062] see figure 1 Describe the water treatment process. Put 20ml of the above-mentioned phenol solution and 2mg supported Au (1.5wt%, dAu: 3.5nm) / TiO 2 Catalyst (British London World Gold Association provides), this reactor is put into the condenser 2 that stirs continuously with magnetic stirrer 3, and wherein the rotating speed of magnetic stirrer is 400 rev / mins, and reaction condition is pH=6.8, T= 70° C.; then, intermittently add 1 ml of hydrogen peroxide solution (30% by weight, Sigma-Aldrich Company, St. Louis, U.S.) to the reactor through the glass stopper 4, and the time intervals are 0, 10 minutes, 20 minutes, 30 minutes, respectively. minutes and 50 minutes, the reaction time is 60-120 minutes; during the test, the degradation rate of phenol is about 30% in 10 minutes, about 40% in 30 minutes, and about 45% in 60 minutes; The instrument used for the dete...
Embodiment 2
[0064] The conditions used in this example are the same as those in Example 1 except that the usage amount of the supported catalyst is increased from 2.0 mg to 10.0 mg. With the increase of the amount of catalyst used, the degradation rate of phenol decreased from about 90% to about 31% when the reaction time was 60 minutes. This may be due to the increase in free radical scavenging rate caused by the decomposition of free radicals (hydroxyl radicals) into hydroxide ions by the catalyst. Therefore, as the amount of catalyst increases, the free radicals capable of oxidizing phenol will decrease, resulting in a decrease in the degradation rate of phenol.
Embodiment 3
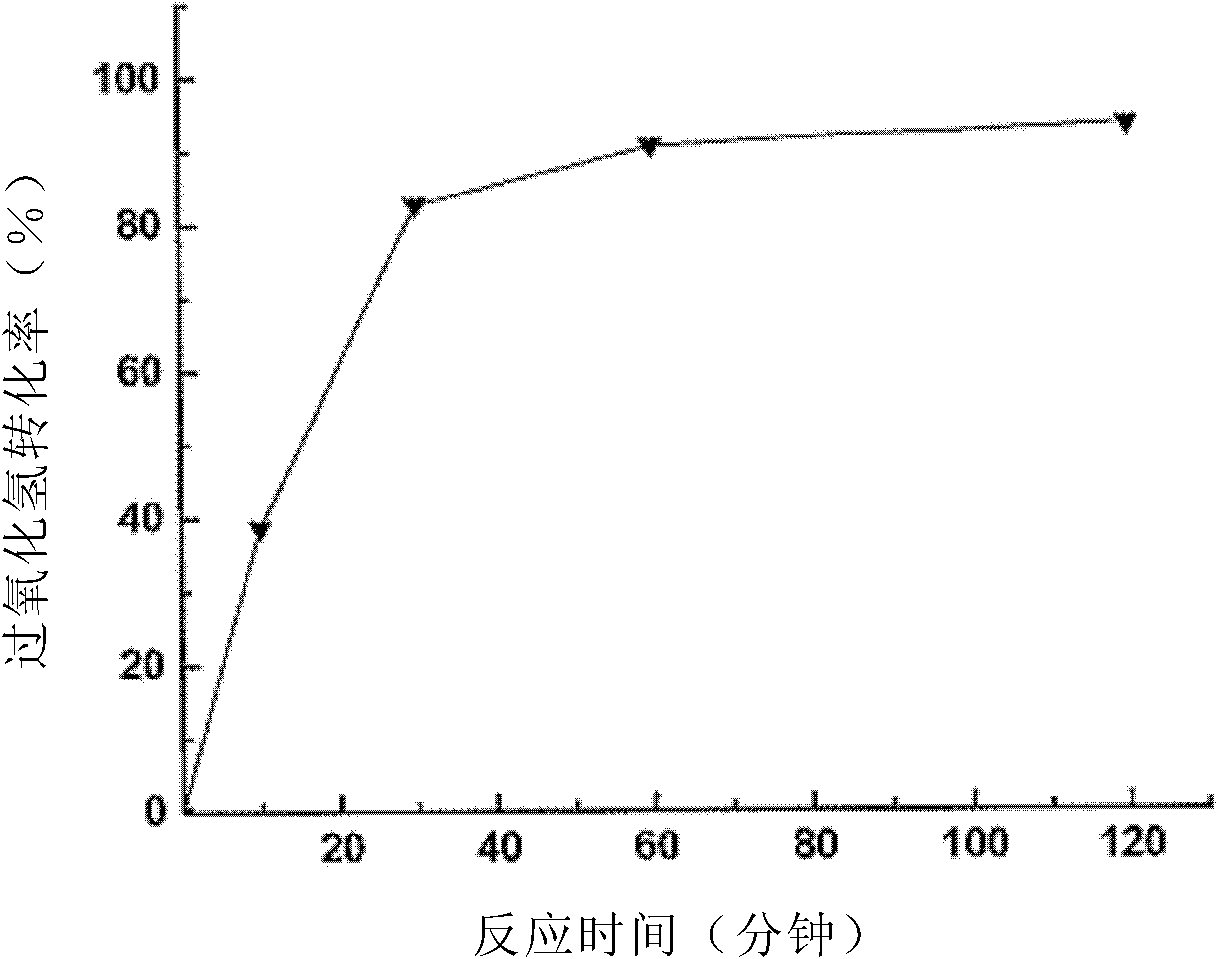
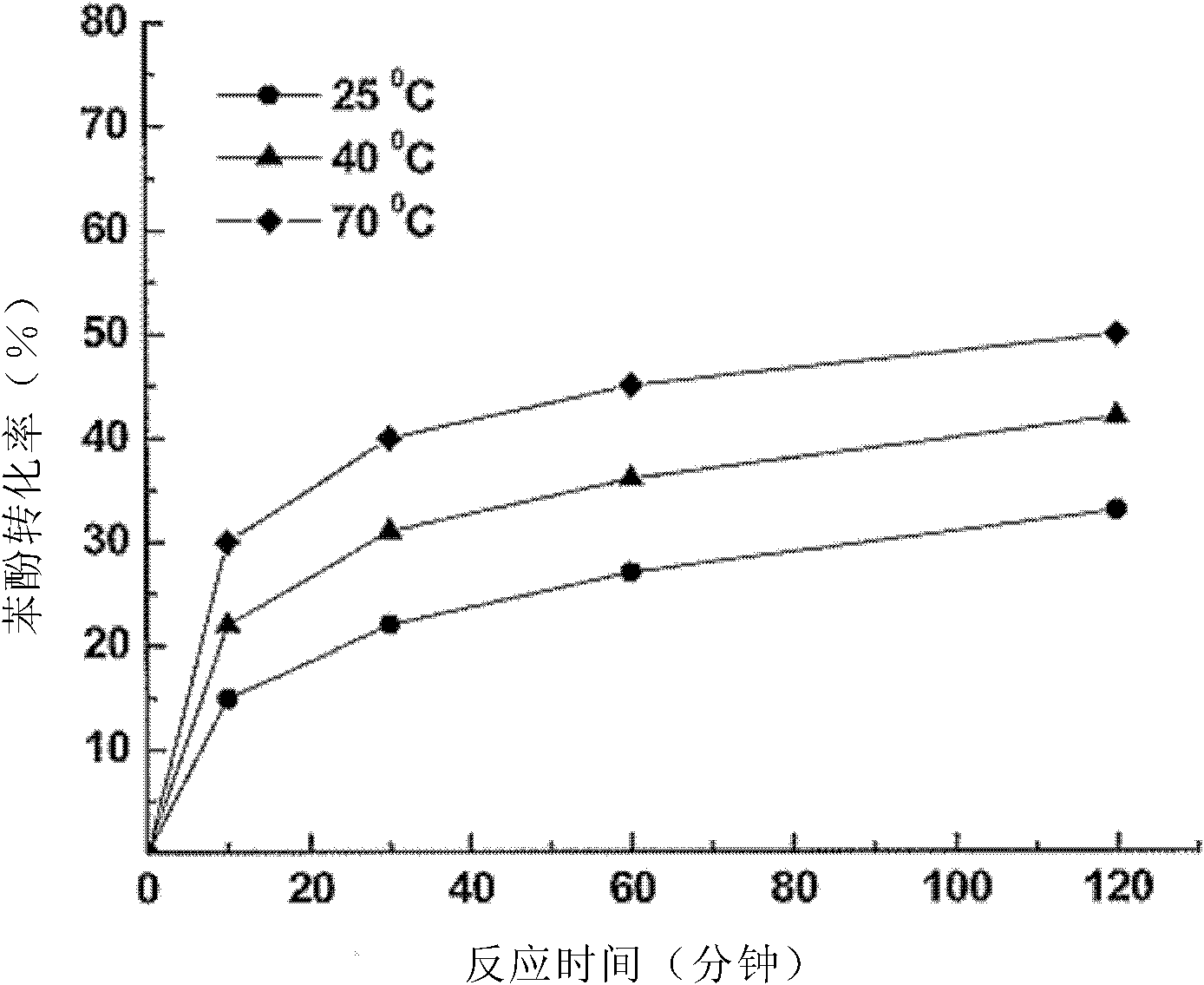
[0066] The conditions of this example are the same as Example 1 except for two additional temperature values of 25°C and 40°C. The result is as image 3 shown. The reaction time was 60 minutes, and when the temperature rose from 25°C to 40°C until reaching 70°C, the conversion rates of hydrogen peroxide were 27%, 35% and 45%, respectively. Therefore, increasing the temperature is beneficial to increase the conversion rate of hydrogen peroxide, so that the free radicals that can be used to oxidize phenol increase, thereby increasing the degradation rate of phenol. Using Au (2.4% by weight) / Hap as a catalyst under the same temperature (298K, 313K, 343K) has done another temperature test, the results are as follows Figure 4 Shown: the reaction time is 120 minutes, the conversion rate of phenol is 50% at 298K; the conversion rate of phenol is 70% at 313K; and its conversion rate is 83% at 343K. Therefore, it can be seen that the reaction speeds up significantly with increasi...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| Specific surface area | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com