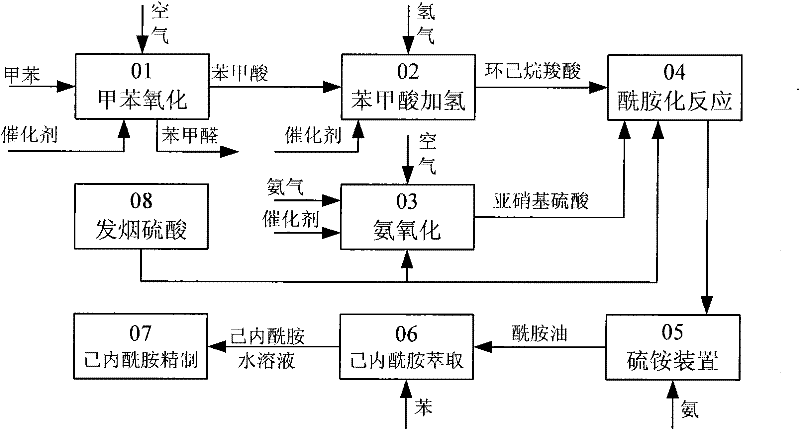
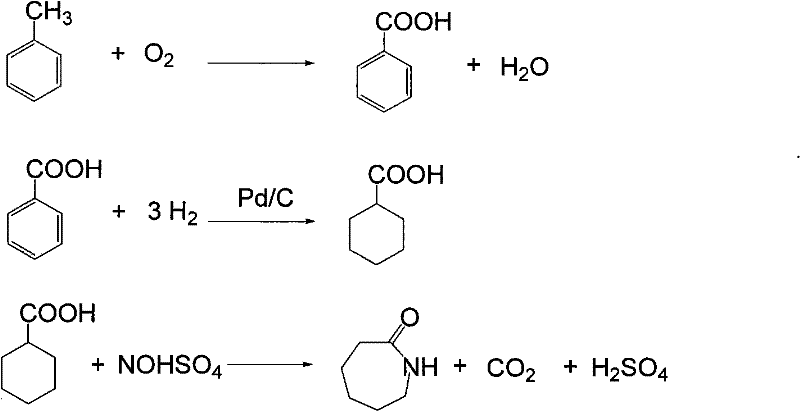
Method for producing caprolactam by methylbenzene
A technology for caprolactam and toluene, applied in the field of caprolactam production, can solve the problems of huge investment cost, huge operating cost, troublesome separation of product and catalyst, alarming catalyst consumption, etc., so as to prolong the operation period, reduce the consumption of precious metal catalyst, and reduce the construction and operation of the device. effect of cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0055] Example 1. Oxidation reaction
[0056] 300 grams of toluene, 700 grams of methyl benzoate returned from the distillation process, 2 grams of catalyzer, oxidized with air in an oxidation kettle, at 160 ° C, under 1Mpa conditions, oxidized toluene to prepare benzoic acid, and cooled the reactant after 2 hours of reaction , Reaction result and the reaction comparison result that does not add methyl benzoate see the table below.
[0057] Table 1. Toluene Oxidation Reaction
[0058] project
Embodiment 2
[0059] Embodiment 2. Esterification reaction
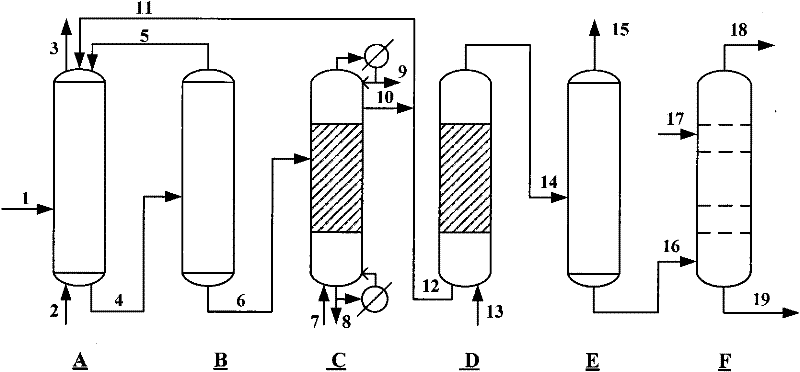
[0060] 6350g of toluene oxidation product enters from the upper part of the esterification tower, and 120g of methanol steam 7 is introduced from the bottom of the esterification tower. The temperature of the tower bottom is kept at 190°C, and the pressure inside the tower is kept at 3.4Mpa. The benzoic acid completely generates methyl benzoate in the tower. About 30 grams of unreacted methyl alcohol are discharged from the tower top condenser 9, 40 g of heavy by-products generated in the oxidation reaction process and catalysts are discharged from the tower kettle 8, and 400 g of methyl benzoate 10 generated by the esterification reaction are discharged from the top tower of the esterification tower Plate mining, wherein 50g of methyl benzoate is circulated back to the oxidation reactor 11, and 350g of methyl benzoate enters the hydrogenation reaction section 12.
Embodiment 3
[0061] Embodiment 3. hydrogenation reaction
[0062] 350g of methyl benzoate 12 and hydrogen 13 from the circulating compressor, at 160°C and 2.5Mpa hydrogen pressure, the hydrogen flow rate is 300L / h, after 1.5 hours of reaction, methyl benzoate is converted into methyl cyclohexyl formate, The hydrogenation product enters the gas-liquid separator, the hydrogenation tail gas 15 is recycled and compressed back to the hydrogenation reactor after removing CO, and 360 g of methyl cyclohexyl carboxylate 16 enters the amidation reactor.
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| boiling point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com