Method for preparing porous hydrogen storage material
A hydrogen storage material, solvothermal synthesis technology, applied in chemical instruments and methods, alkali metal oxides/hydroxides, inorganic chemistry, etc., can solve problems such as difficulty in applying vehicle-mounted hydrogen fuel cells, poor hydrogen storage performance, etc. , to achieve good hydrogen storage effect and good hydrogen storage effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Add 0.3mmol of 4,4'-biphenyldicarboxylic acid to the reaction vessel, continue to add 4.0mL of N,N'-dimethylacetamide to dissolve, then add 2.0mL of absolute ethanol, 0.3mL of 0.4mol / L piperazine solution and 0.2mL 2.7mol / L nitric acid solution, after stirring for 20min, add 0.2mmol indium chloride, continue stirring for 20min, then transfer the reaction vessel to a dry box and heat up to 85°C, and continue to heat up to Keep at 105°C for 48 hours, then slowly cool down to 60°C at a rate of 5°C / h, and finally cool naturally to room temperature.
[0033] The mixture in the reaction vessel was filtered, washed with absolute ethanol, and evaporated to dryness naturally to obtain crystals with a yield of about 63%.
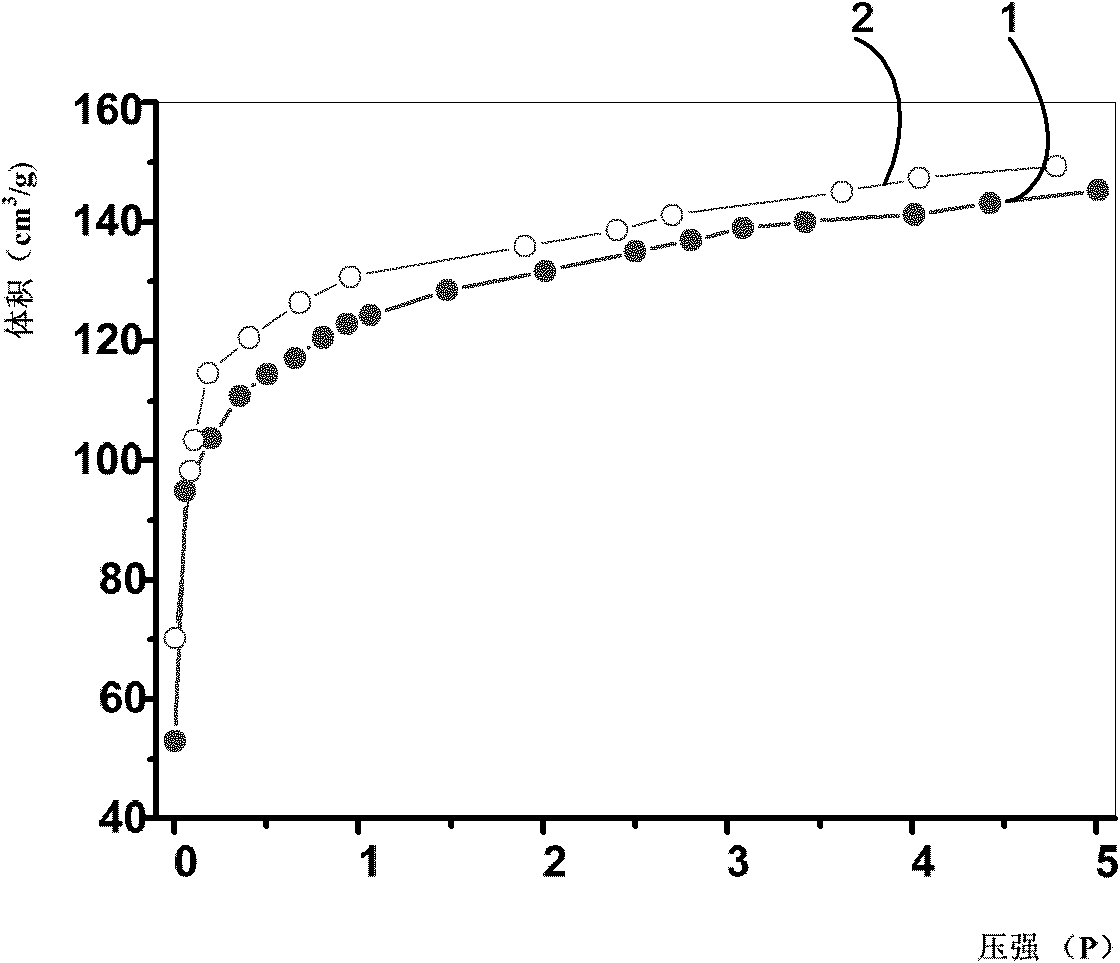
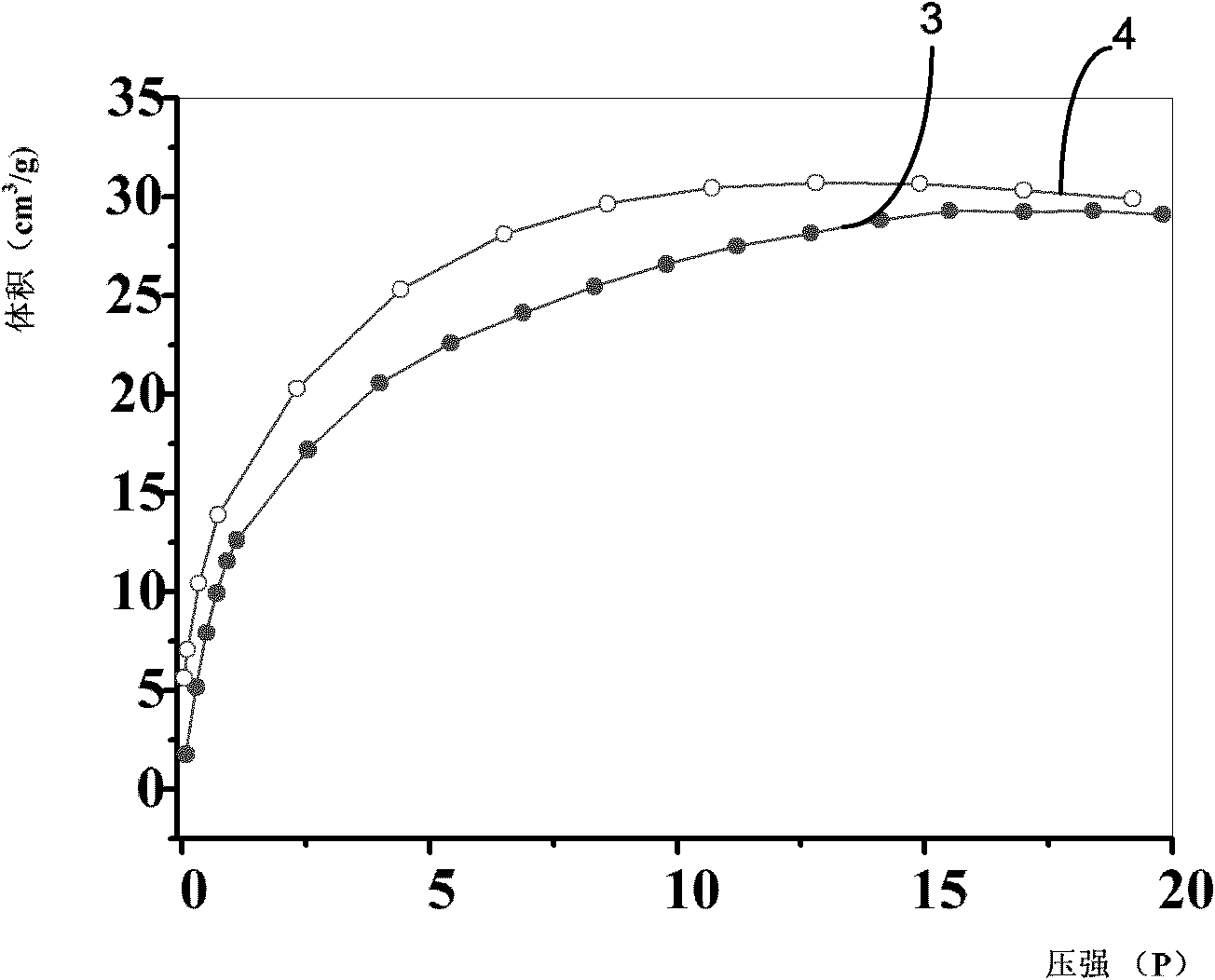
[0034] see figure 1 Shown is the isothermal curve of the hydrogen storage material prepared in this example at -196°C for absorbing and releasing hydrogen. Curve 1 is the relationship between the volume and pressure of hydrogen absorbed by the hydrogen storag...
Embodiment 2
[0037]Add 0.2mmol of 2,6-naphthalene dicarboxylic acid to the reaction vessel, continue to add 3.0mL of N,N'-dimethylacetamide to dissolve it, then add 2.0mL of absolute ethanol, 0.3mL of 0.4mol / L Piperazine solution and 0.4mL 2.7mol / L nitric acid solution, stirred for 20min, then added 0.2mmol indium nitrate, continued to stir for 20 minutes, then transferred the reaction vessel to a dry box and raised the temperature to 85°C, and continued to heat up to 105°C after 24h of reaction ℃ and kept for 48h, then slowly cooled to 60℃ at a rate of 5℃ / h, and finally cooled to room temperature naturally.
[0038] The mixture in the reaction vessel was filtered, washed with absolute ethanol, and evaporated to dryness naturally to obtain crystals with a yield of about 46%.
Embodiment 3
[0040] Add 0.15mmol of 3,3',4,4'-biphenyltetracarboxylic acid into the reaction vessel, continue to add 4.0mL of N,N'-dimethylacetamide to dissolve it, and then add 5.0mL of absolute ethanol in sequence , 0.4mL 0.4mol / L piperazine solution and 0.4mL 2.7mol / L nitric acid solution, after stirring for 20min, add 0.3mmol indium sulfate, continue stirring for 20 minutes, then transfer the reaction vessel to a dry box and heat up to 85°C, react After 24 hours, continue to raise the temperature to 105°C and maintain it for 48 hours, then slowly cool down to 60°C at a rate of 5°C / h, and finally cool down to room temperature naturally.
[0041] The mixture in the reaction vessel was filtered, washed with absolute ethanol, and evaporated to dryness naturally to obtain crystals with a yield of about 49%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com