Electron deficient olefins and curable compositions prepared therefrom
A compound and composition technology, applied in the field of new electron-deficient olefins, can solve the problems that new electron-deficient olefins have not been described before
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
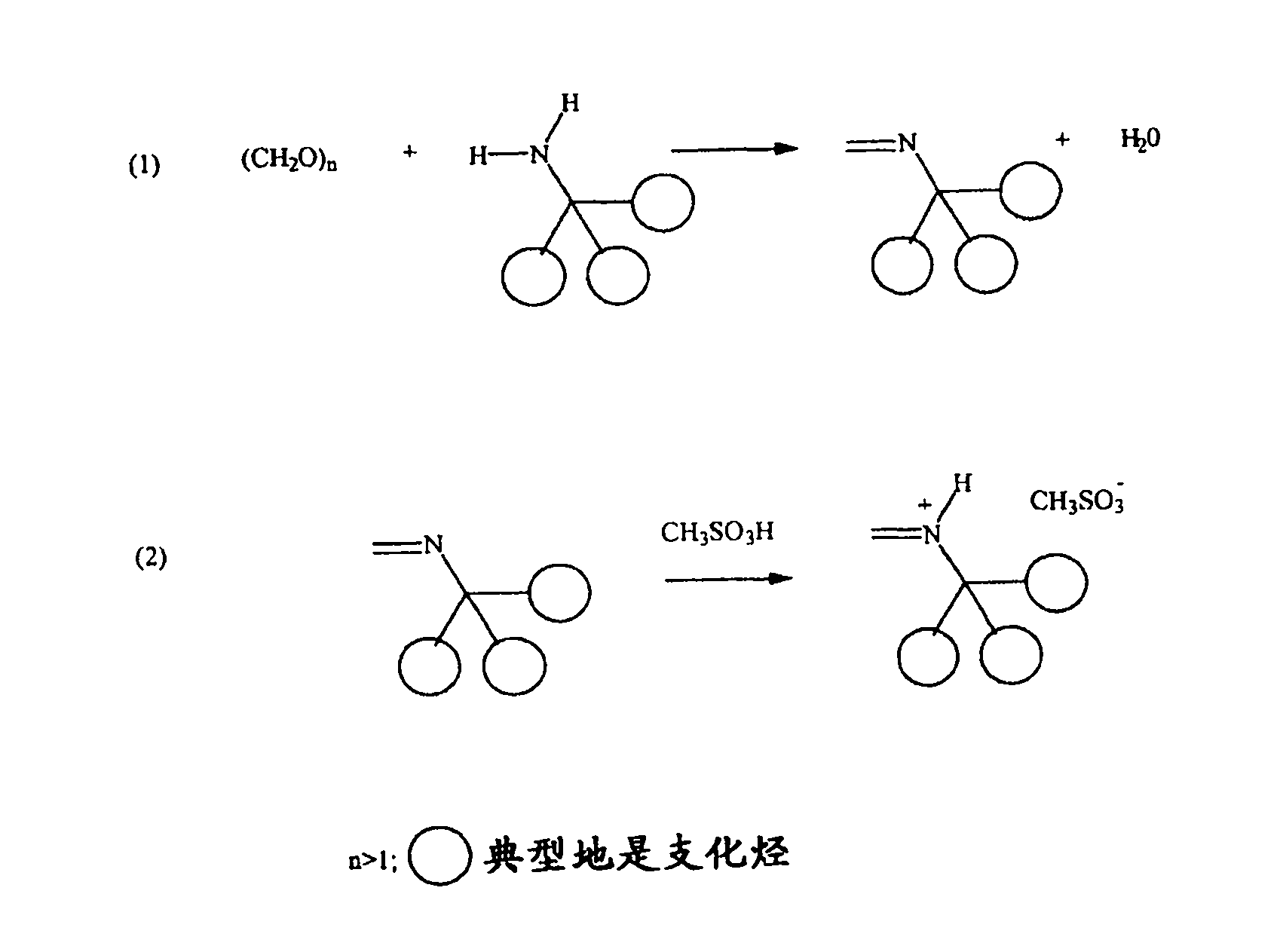
[0160] PRIMENE 81-R imines are prepared by reacting PRIMENE 81-R amines with a stoichiometric equivalent of paraformaldehyde and removing the water of condensation. All imines formed are distillable liquids and exist as stable monomeric imines as 1 H NMR 60 MHz (CDCl 3 )2H s(br)7.45ppm and FTIR(1650cm -1 ) as confirmed.
Embodiment 2
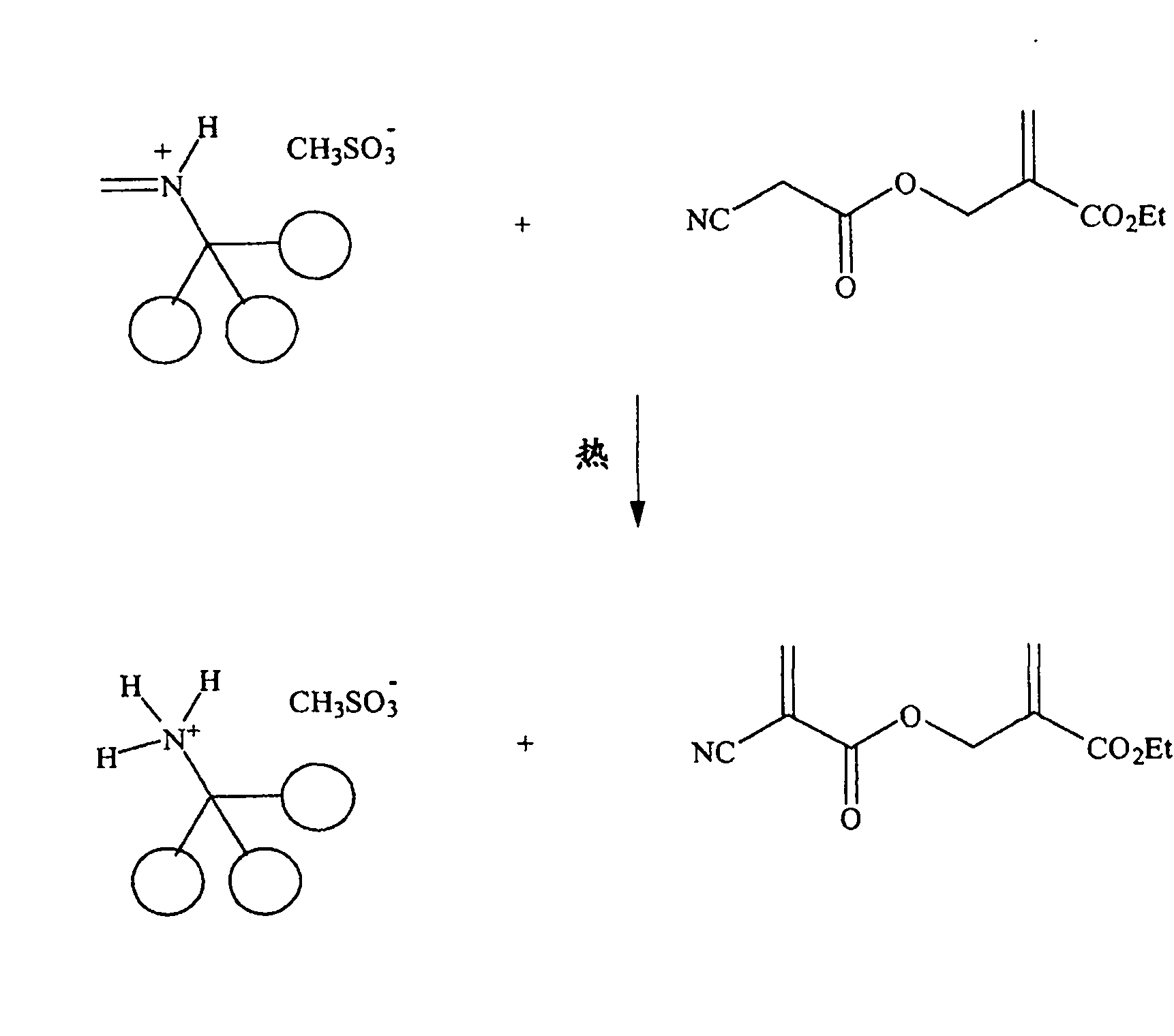
[0162] PRIMENE 81-R iminium-MSA was prepared from PRIMENE 81-R imine by dropwise addition of methanesulfonic acid with stirring at ice-water bath temperature, resulting in a pale yellow iminium salt.
Embodiment 3
[0164]
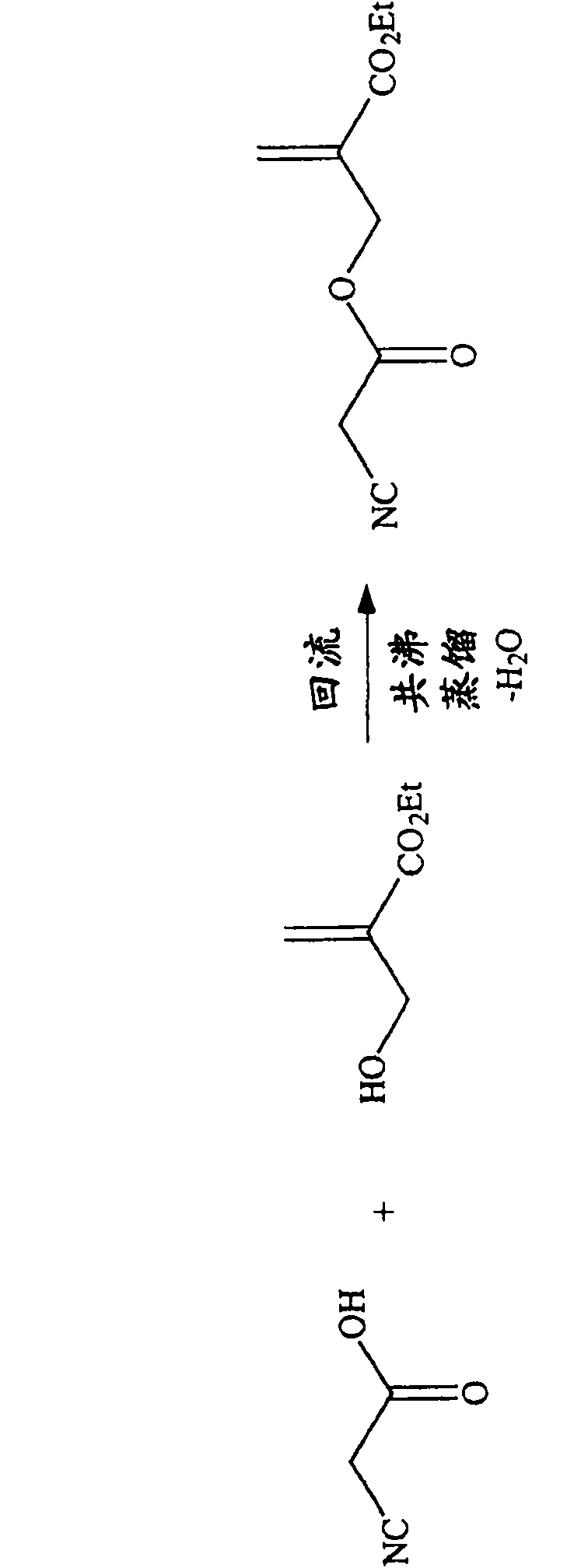
[0165] To a stirred mixture of cyanoacetic acid (90 g, 1.05 mol), ethyl 2-hydroxymethacrylate (130 g, 1.0 mol), p-toluenesulfonic acid (500 mg) and hydroquinone (200 mg) was added toluene (150 mL), and The mixture was refluxed at a temperature of 150°C to remove water azeotropically.
[0166] After cooling, the reaction product was washed successively with 30% brine and water. The organic layer was dried over anhydrous sodium sulfate, filtered and the solvent was removed by rotary evaporator. The crude reaction product was purified by vacuum distillation (120-126° C. / 0.2 mbar) and the ester of structure A (102 g, 0.52 mol) was isolated in 52% yield. 1 H NMR (60MHz, CDCl 3): δ6.39(s, 1H), 5.89(s, 1H), 4.90(s, 2H), 4.28(q, J=6.0Hz, 2H), 3.50(s, 2H), 1.32(t, J= 6.0Hz, 3H); FT-IR (film): 2983.3, 2935.3, 2264.3, 1753.6, 1719.7, 1640.0, 1448.3, 1368.2, 1310.3, 1177.0, 1027.1, 817.2cm -1 ; GC / MS (EI) m / z (%): 198 (2) [M + +H], 152(40), 129(25), 101(38), 85(100), 83(4...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com