Application of chitosan oligosaccharide in preparation of medicines for preventing and treating endotoxemia/bacteremia
A technology of endotoxemia and chitosan oligosaccharide, applied in medicine or health food, the application field of chitosan oligosaccharide in the prevention and treatment of endotoxemia/bacteremia, can solve clinical application limitations, damage , toxic and side effects, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
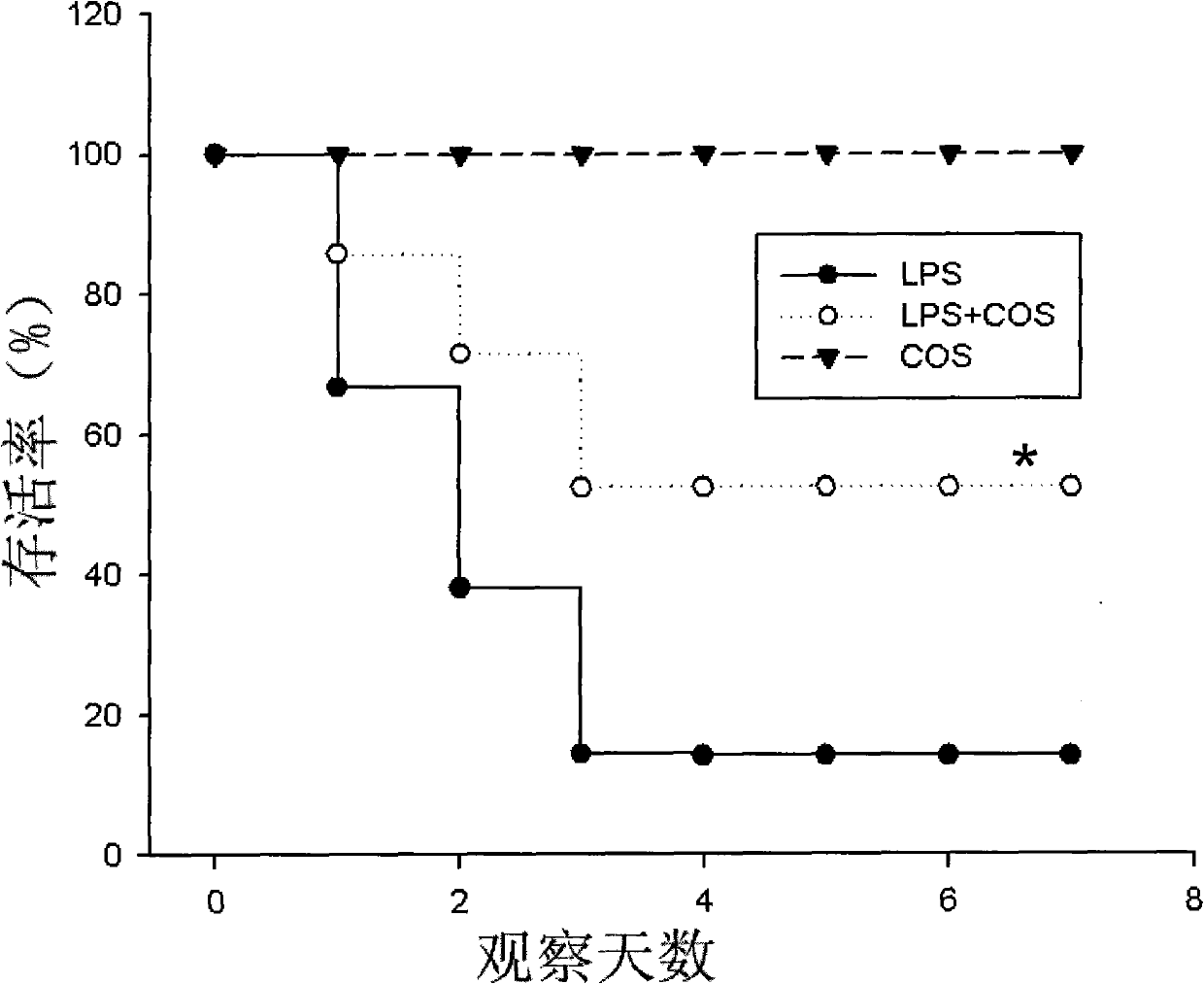
[0023] Reference literature (Aoshiba, K., Onizawa, S., Tsuji, T., & Nagai, A. (2009). Therapeutic effects of erythropoietin in murine models of endotoxin shock. CRITICAL CARE MEDICINE, 37(3), 889-898 ), 24 mice (8 weeks, 18-22 g, half male and half male) were divided into one group, a total of 3 groups, and the drugs were injected intraperitoneally. Divided into simple chitooligosaccharide group (deacetylation degree ≥ 95%, molecular weight < 1000Da) (COS), endotoxin group (LPS), chitooligosaccharide (deacetylation degree ≥ 95%, molecular weight < 1000Da) treatment group, 100mg / kg Body weight (LPS+COS). Continuous observation for 7 days, every 6 hours for the first 3 days, and every 12 hours for the next 4 days.
[0024] figure 1 In the figure, the solid dots represent the endotoxin LPS group, the hollow dots represent the LPS+COS treatment group, and the inverted triangles represent the simple chitosan oligosaccharide group. It can be seen from the figure that 100 mg / kg bo...
Embodiment 2
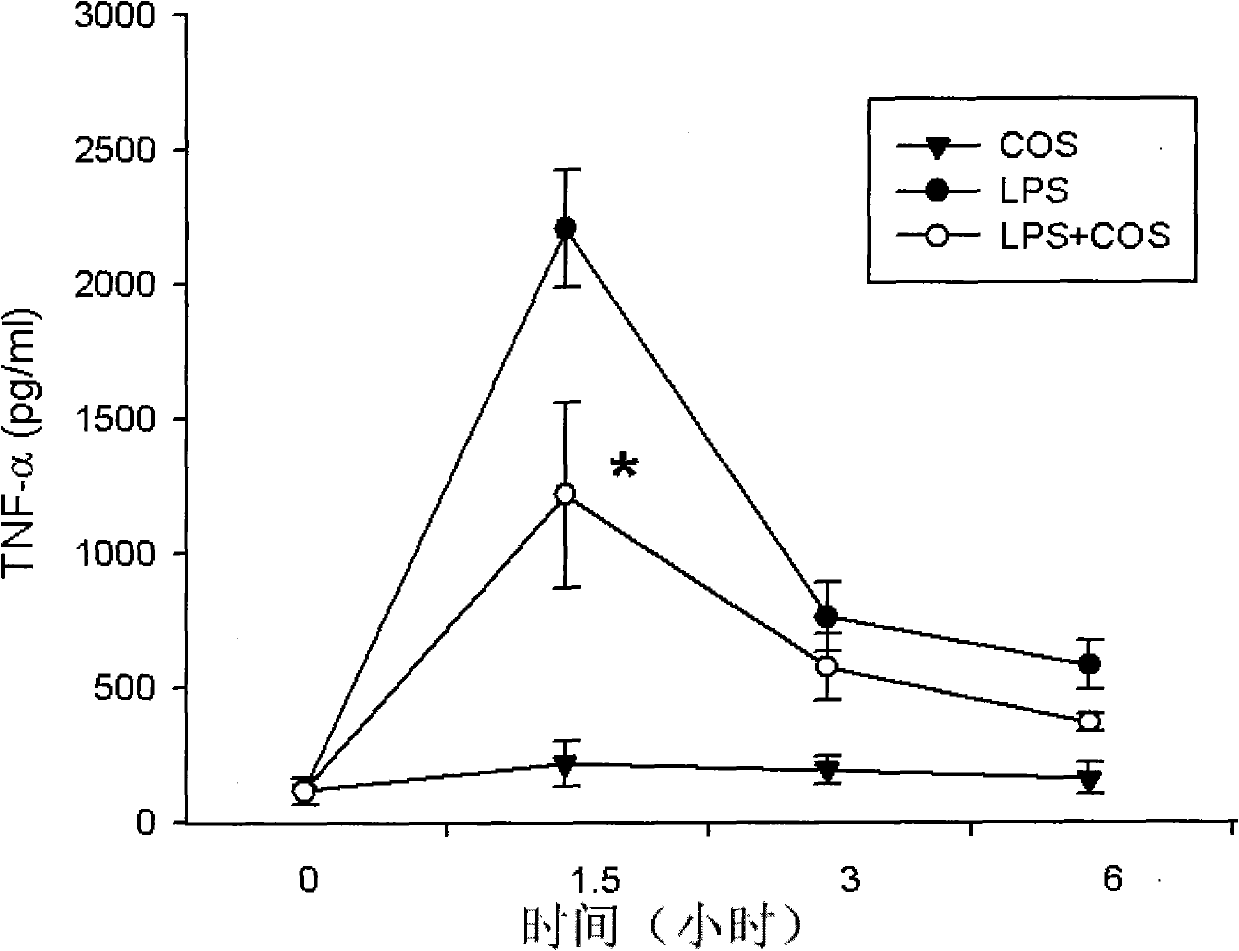
[0026] More than 12 mice were divided into 3 groups, and the drugs were injected intraperitoneally. Simple chitosan oligosaccharide (deacetylation degree ≥ 95%, molecular weight < 1000Da) group, LPS group (LPS), chitosan oligosaccharide (deacetylation degree ≥ 95%, molecular weight < 1000Da) treatment group (100mg / kg body weight), respectively Blood was collected from 6 mice at 1.5 and 3 hours after LPS injection. Mouse serum was separated for TNF-α ELISA analysis. Briefly, 100 μl of diluted serum sample was added to the bottom of the well of the ELISA plate, incubated at 37°C for 2 hours, 350 μl of washing solution was manually washed 5 times, and 100 μl of enzyme-labeled antibody was added, incubated at 37°C for 2 hours, and the plate was washed manually with 350 μl of washing solution 5 times, add 100 μl of substrate working solution and incubate at 37°C for 30 minutes, then add 100 μl of stop solution, and read the plate at 450 nm on a microplate reader.
[0027] figur...
Embodiment 3
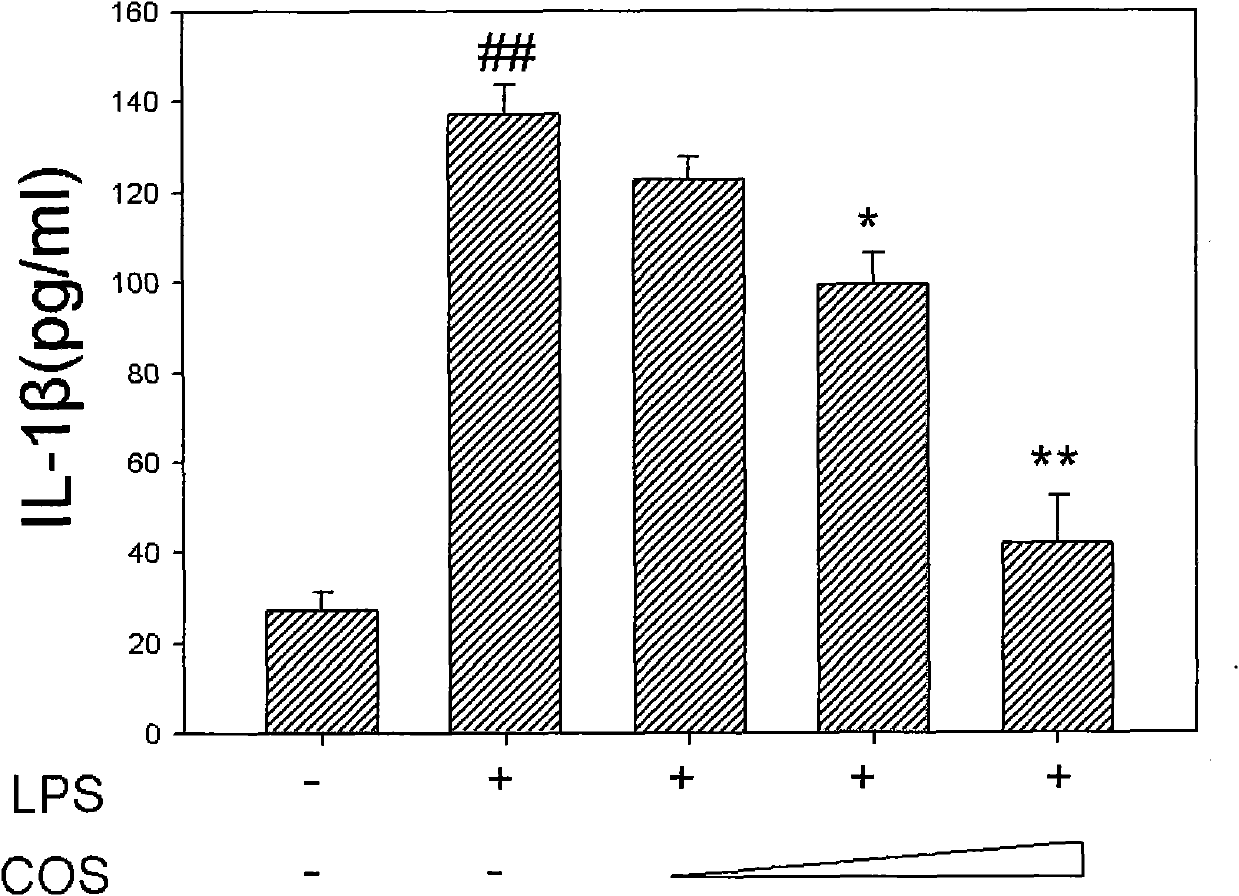
[0029]The mouse peritoneal cell line RAW264.7 was pressed at 0.1-10×10 6 pcs / mL (in this example, 1-2×10 6 seed / mL) in a 6-well plate, cultivated for 10-24h (this example uses 12h), then add chitosan oligosaccharide (deacetylation degree ≥ 95%, molecular weight < 1000Da) into the culture medium, the final concentration is respectively 100μg / mL, 200μg / mL, 400μg / mL, add LPS (100ng / ml) at the same time, continue to culture for 6h, discard culture medium, wash with PBS (pH7.4) for 3 times, add ELISA specimen diluent, liquid nitrogen repeatedly The cells were lysed by freezing and thawing, and the lysate was taken for ELISA to detect the expression of pro-IL-1β in the cells, and the steps were the same as above.
[0030] image 3 From left to right, respectively, blank group, LPS group, LPS+COS and COS concentration gradually increased. It can be seen from the figure that 400 μg / mL chitosan oligosaccharide can significantly inhibit LPS-mediated cell secretion of inflammatory fac...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com