Application of optimized TACI (Transmembrane Activator and CAML Interactor)-Fc fusion protein in preparation of medicaments for treating rheumatoid arthritis
A fusion protein, rheumatoid technology, used in drug combinations, antipyretics, anti-inflammatory agents, etc., can solve problems such as easy protein degradation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
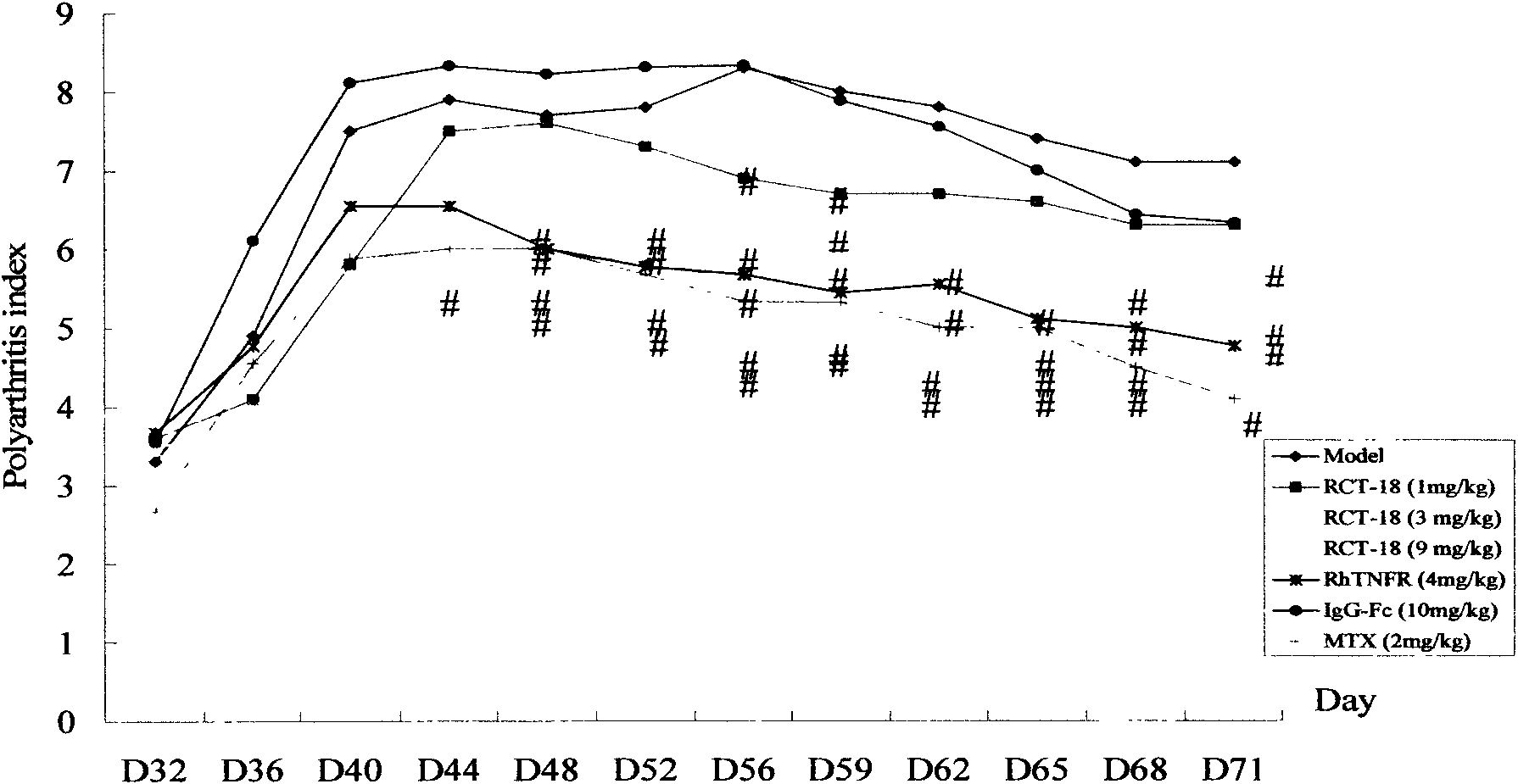
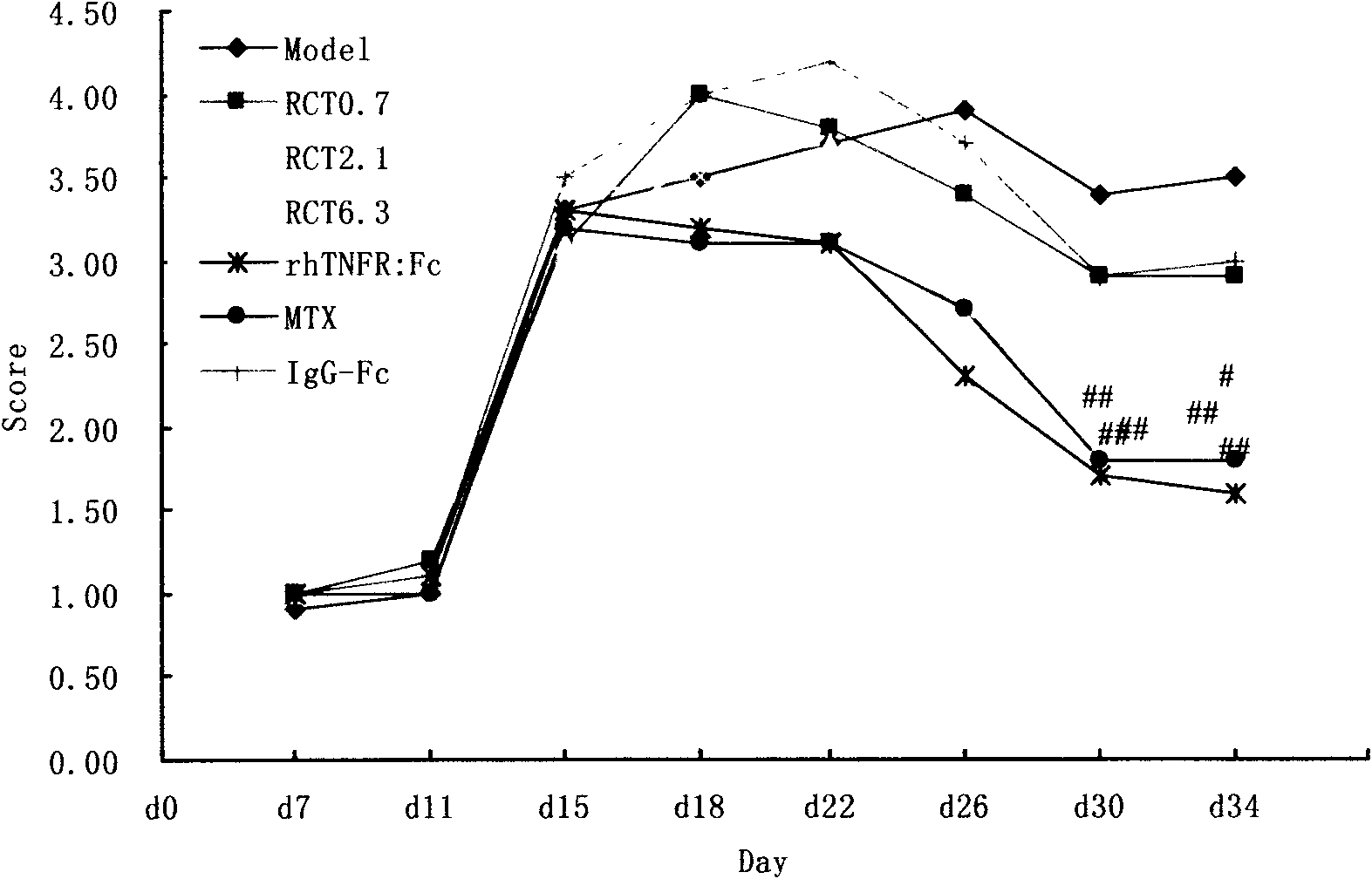
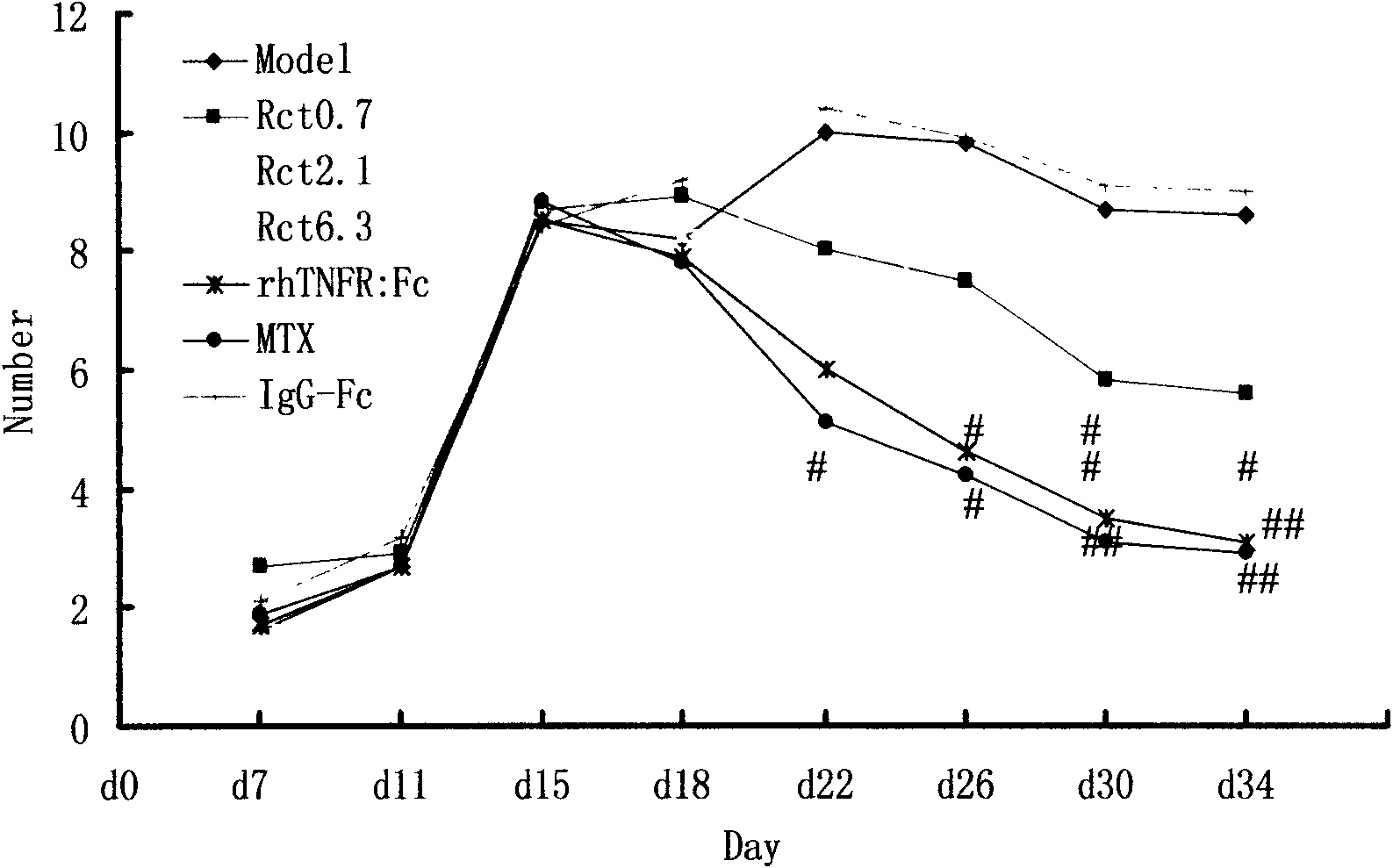
[0039] Example 1: The main pharmacodynamic study of RCT-18 on collagen-induced arthritis (CIA) in mice and adjuvant-induced arthritis (AA) in rats.
[0040] 1.1 Test method
[0041] ① Pharmacodynamic test method on CIA mice
[0042] On d0 and d21, DBA / 1 mice were intradermally injected with 100 μg type II collagen and complete Freund's adjuvant at multiple points on the base of the tail and back to induce the CIA mouse model, and were randomly divided into 8 groups, namely normal group, model group, RCT-18 Three dose groups (1, 3, 9mg / Kg, subcutaneous injection, once every two days, medication for 6 weeks), positive control group Yisaipu (rhTNFR:Fc, 4mg / Kg, subcutaneous injection, once every two days, medication for 6 weeks) ), methotrexate (MTX, 2mg / Kg, intragastric administration, once a week) group, and an IgG-Fc (9mg / Kg, subcutaneous injection, once every two days, medication for 6 weeks) negative control group. Normal group and model group were injected with normal sali...
Embodiment 2
[0068] Example 2: Pharmacokinetic study of RCT-18 in rats and rhesus monkeys
[0069] 2.1.1 Pharmacokinetic study in rats
[0070] This study established the RCT-18 in plasma 125 I isotope labeling tracer assay method, and application established method, studied rat intravenous injection (iv) 1mg / kg and subcutaneous injection (sc) three single-dose groups (1,4 and 16mg / kg) and multiple ( 5 times) After subcutaneously injecting a dose group (4mg / kg) of RCT-18 lyophilized injection, the time-dependent change curve of RCT-18 in plasma was analyzed by using DAS2.0 software, compartment model and statistical moment method respectively. academic analysis. Results: ①The CPM value of blank plasma was low and a stable value. The extraction recovery rate of RCT-18 in plasma is greater than 79%, the method recovery rate is greater than 99%, and the inter-assay and intra-assay coefficients of variation are both less than 10%. The linear range of RCT-18 was 0.1-20 μg / ml, and the correl...
Embodiment 3
[0083] Embodiment 3: RCT-18 animal safety evaluation study
[0084] In each test of non-clinical animal safety evaluation, RCT-18 showed good tolerance and safety in all test animals, and the test results are shown in Table 3.
[0085] Table 3 RCT-18 non-clinical safety test results
[0086]
[0087] In summary, the present invention provides an optimized TACI-Fc fusion protein for the preparation of the application of drugs for the treatment of rheumatoid arthritis, the drug of the present invention is safe and effective for the treatment of rheumatoid arthritis, and is beneficial to reduce The cost of this type of recombinant protein drug is suitable for industrial promotion and application.
[0088]
[0089]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com