High-reliability vanadium ion electrolyte
A technology with high stability and electrolyte, which is applied in the direction of regenerative fuel cells, etc., can solve the problems of battery efficiency reduction, ethanol oxidation, and failure to meet the requirements, so as to improve charge and discharge performance and cycle stability, improve stability, and inhibit precipitation Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] Potassium dihydrogen phosphate: analytically pure;
[0026] Sodium sulfate: analytically pure.
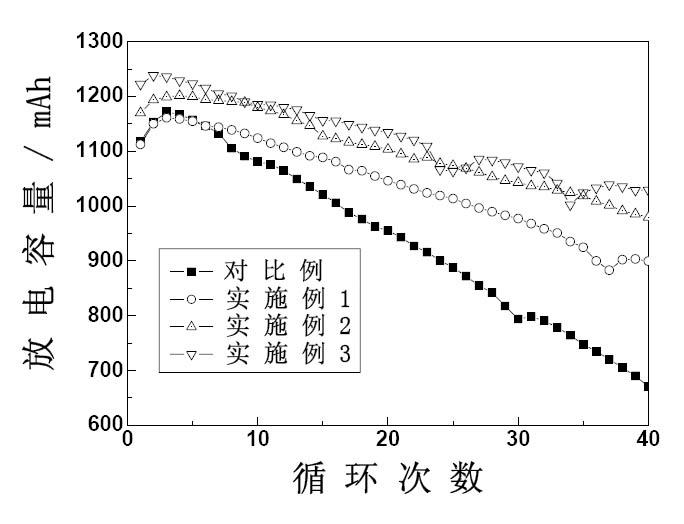
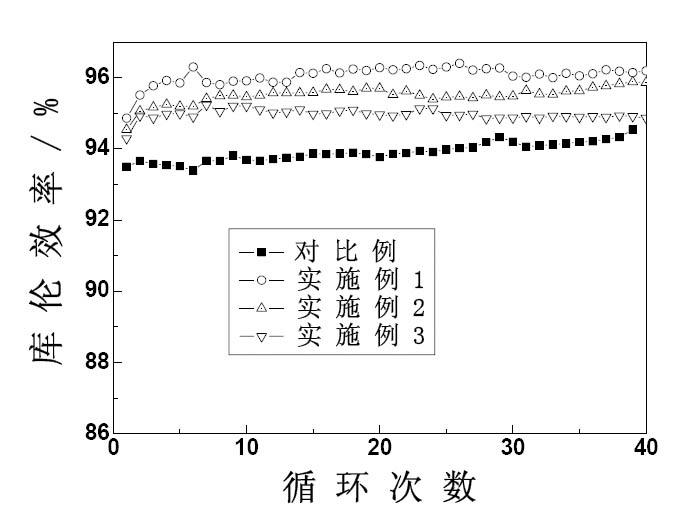
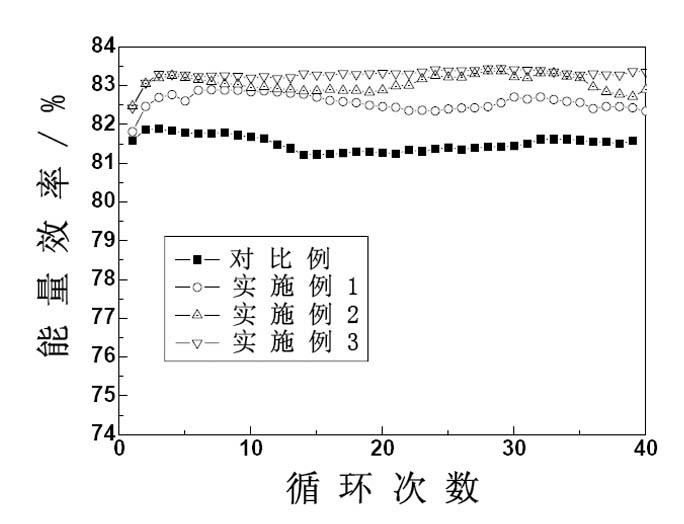
[0027] Take the total vanadium concentration as 1.4 molL -1 , the concentration of sulfuric acid is 2.8molL -1 V(IV):V(III)=1:1 blank (without additives) vanadium ion electrolyte 80mL, add 0.1g potassium dihydrogen phosphate and 2g sodium sulfate, divide the obtained electrolyte into two parts, respectively Place it in the positive and negative liquid storage tanks of the simulated vanadium battery for charge and discharge experiments. The experimental results are shown in Table 1.
Embodiment 2
[0029] Phosphoric acid: analytically pure;
[0030] Polyethylene glycol: analytically pure.
[0031] Take the total vanadium concentration as 1.4 molL -1 , the concentration of sulfuric acid is 2.8molL -1 V(IV):V(III)=1:1 blank (without additives) vanadium ion electrolyte 40mL, add 0.1mL 1molL -1 h 3 PO 4 solution, placed in the positive storage tank of the simulated vanadium battery, and the total vanadium concentration was 1.4 molL -1 , the concentration of sulfuric acid is 2.8molL -1 V(IV):V(III)=1:1 blank (without additives) vanadium ion electrolyte 40mL, add 0.1g polyethylene glycol, place in the negative electrode storage tank of the simulated vanadium battery, and charge and discharge experiment. The experimental results are shown in Table 1.
Embodiment 3
[0033] Sodium sulfate: analytically pure;
[0034] CTAB: analytically pure;
[0035] Glycerol: analytically pure;
[0036] Sodium hexametaphosphate: analytically pure.
[0037] Take the total vanadium concentration as 1.4 molL -1 , the concentration of sulfuric acid is 2.8molL -1 V(IV):V(III)=1:1 blank (without additives) vanadium ion electrolyte 40mL, add 3g sodium sulfate, 0.01g CTAB, place in the positive electrode storage tank of the simulated vanadium battery, take the total Vanadium concentration is 1.4molL -1 , the concentration of sulfuric acid is 2.8molL -1 V(IV):V(III)=1:1 blank (without additives) vanadium ion electrolyte 40mL, add 0.1g sodium hexametaphosphate, 1g glycerol, place in the negative storage tank of the simulated vanadium battery In, charge and discharge experiments were carried out. The experimental results are shown in Table 1.
[0038] figure 1 , figure 2 and image 3 The relationship curves of discharge capacity, coulombic efficiency, en...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com